What Is Energy in Molecules?
You have already learned about energy in everyday life such as kinetic energy, potential energy, energy needed to do work, energy from food, and many others. These ideas all remind us of one simple point:
Energy makes things happen.
But in chemistry, we are not looking at moving cars or falling objects.
Instead, we look much deeper at the energy inside atoms and molecules themselves. So, where exactly is that energy stored?
How Do Molecules Store Energy?
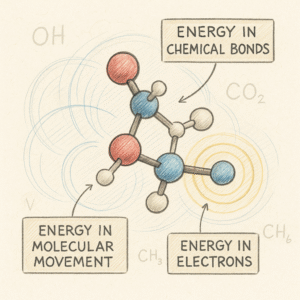
Even though molecules are far too small to see, they are full of activity and structure. They store energy in three main ways:
1. Energy in chemical bonds
Atoms in a molecule are joined by chemical bonds, and these bonds contain energy. This happens because:
-
When a bond forms, energy is released as the atoms settle into a more stable arrangement.
-
When a bond breaks, energy must be supplied to pull the atoms apart.
Because forming and breaking bonds always involve energy, we say that the bonds themselves store chemical energy.
2. Energy in molecular movement
Molecules are never completely still:
-
In solids, they vibrate.
-
In liquids and gases, they rotate and move around more freely.
This motion contributes to the total energy of the molecule.
3. Energy in electrons
Electrons occupy different energy levels around the nucleus.
Electrons in higher levels or more spread-out arrangements carry more energy, adding to the molecule’s overall chemical energy.
When chemists refer to chemical energy, they are really talking about the combination of these two things:
-
The energy stored in chemical bonds
-
The energy in the movement and arrangement of atoms and electrons
Understanding this stored energy is essential, because its changes affect everything that happens during a reaction.
Why Does This Matter in Chemistry?
Chemical energy becomes important when substances react. During a reaction:
-
Bonds in the reactants break → this needs energy
-
New bonds form in the products → this releases energy
Every reaction must involve some energy change, either going into the system or out of it. This is why some reactions feel warm and others feel cold. Later in this topic, we will look closely at exothermic reactions (energy given out) and endothermic reactions (energy taken in). For now, you only need to understand that reactions always involve changes in chemical energy.
A Quick Note on Energy Conservation
To understand energy changes properly, we rely on a key scientific rule:
Energy cannot be created or destroyed. It can only be transferred or converted.
In chemistry, this means:
-
Energy used to break bonds must come from somewhere.
-
Energy released when bonds form must go somewhere.
Nothing disappears. Nothing appears out of nowhere. Energy is simply moved or changed form. Because of this rule, every chemical reaction will always involve energy being transferred between the molecules and their surroundings. This idea connects directly to concepts you will learn next enthalpy, enthalpy changes, and reaction energy diagrams.
What Is Enthalpy?
When we study energy changes in chemistry, we need a clear way to describe the heat stored in substances and the heat transferred during reactions. This is what the idea of enthalpy allows us to do. Enthalpy, written as H, represents the total heat energy stored in a substance or a chemical system. Although we cannot measure the absolute enthalpy of a substance directly, we can measure how much it changes during a reaction and this is the part that truly matters in chemistry.
Chemists rely on enthalpy because reactions almost always involve heat moving in or out. Some reactions give out heat and warm the surroundings, while others take in heat and make the surroundings cooler. Enthalpy gives us a consistent way to describe these heat changes and compare one reaction with another. Whenever substances react, the enthalpy of the products is usually different from the enthalpy of the reactants, and this difference is called the enthalpy change.
What Is Enthalpy Change (ΔH)?
![]()
Enthalpy change, written as ΔH, is the amount of heat energy transferred during a chemical reaction at constant pressure. We use the sign of ΔH to understand how heat moves:
-
ΔH is negative when the reaction releases heat (exothermic).
-
ΔH is positive when the reaction absorbs heat (endothermic).
A simple way to think about this is:
if the products store less energy than the reactants, the extra energy must be released to the surroundings; if the products store more energy, the system must take energy in for the reaction to occur. This makes ΔH a very direct indicator of how the energy of the system has changed.
Units of Enthalpy Change
Enthalpy change is measured in kJ/mol (kilojoules per mole).
This tells us how much heat energy is transferred when one mole of a substance reacts.
Why Do Chemists Mainly Measure Enthalpy?
Molecules contain many forms of internal energy vibrational energy, electron energy, and bond energy so it is natural to ask why chemistry focuses mainly on enthalpy, the heat component. The reason is that when reactions happen, the most meaningful and measurable change is the heat released or absorbed when bonds break and form.
Other types of molecular energy change too, but we cannot measure them directly with ordinary laboratory methods. Heat energy, however, can be measured easily using temperature changes, calorimetry, or heat transfer to water. Because heat is the only part of a molecule’s internal energy that we can observe and measure in a simple, reliable way, enthalpy becomes the most practical and useful quantity for studying reactions.
This focus on enthalpy also tells us exactly what we need to know about reactions. It allows us to determine whether a reaction is exothermic or endothermic, how much energy is involved, and how the temperature of the surroundings will change. It links the invisible world of atoms and bonds to measurable changes in the real world.
Why Is Enthalpy Change Important?
A good understanding of ΔH helps students make sense of what is happening during a reaction. It allows you to see whether a reaction gives out heat or takes heat in, and it helps you understand why the temperature changes during an experiment. Knowing about enthalpy change also makes it easier to read reaction profile diagrams and to understand how bond breaking and bond formation affect energy. These ideas connect directly to real-life processes such as burning fuels, dissolving substances, neutralisation reactions, and even how batteries produce energy. For these reasons, enthalpy change is an essential topic in both GCSE and U.S. high-school chemistry.
In Summary Enthalpy is the heat energy stored in a substance, and enthalpy change is the heat transferred during a reaction. A negative ΔH shows that heat is released, while a positive ΔH shows that heat is absorbed. We focus on enthalpy because heat is the part of a molecule’s internal energy that changes during reactions and the part we can measure easily. This makes enthalpy a key tool for understanding how and why reactions transfer energy.
How Enthalpy Change Is Measured (Calorimetry)
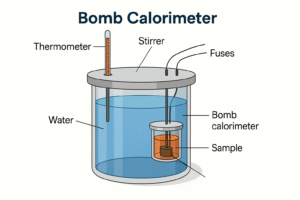
Calorimetry is the technique chemists use to measure enthalpy change (ΔH) in a reaction. You do not need the full method here, but you should understand the basic idea before moving on.
Calorimetry works on a very simple principle:
-
If a reaction releases heat, the temperature of the surroundings increases.
-
If a reaction absorbs heat, the temperature of the surroundings decreases.
-
By measuring this temperature change, we can calculate how much energy has been transferred.
-
The reaction usually takes place in an insulated container so that heat is not easily lost.
-
The key measurements are temperature change, mass of solution, and specific heat capacity.
-
These values allow us to calculate ΔH using the temperature change caused by the reaction.
That is all you need at this point.
The full practical method step-by-step instructions, formulas, diagrams, and worked examples is explained in detail in our separate calorimetry blog.
Endothermic and Exothermic Reactions
Chemical reactions always involve energy being transferred because bonds in the reactants must break and new bonds form in the products. Whether the reaction absorbs heat or releases heat depends on the balance between these steps. This leads to two main types of reactions: endothermic and exothermic.
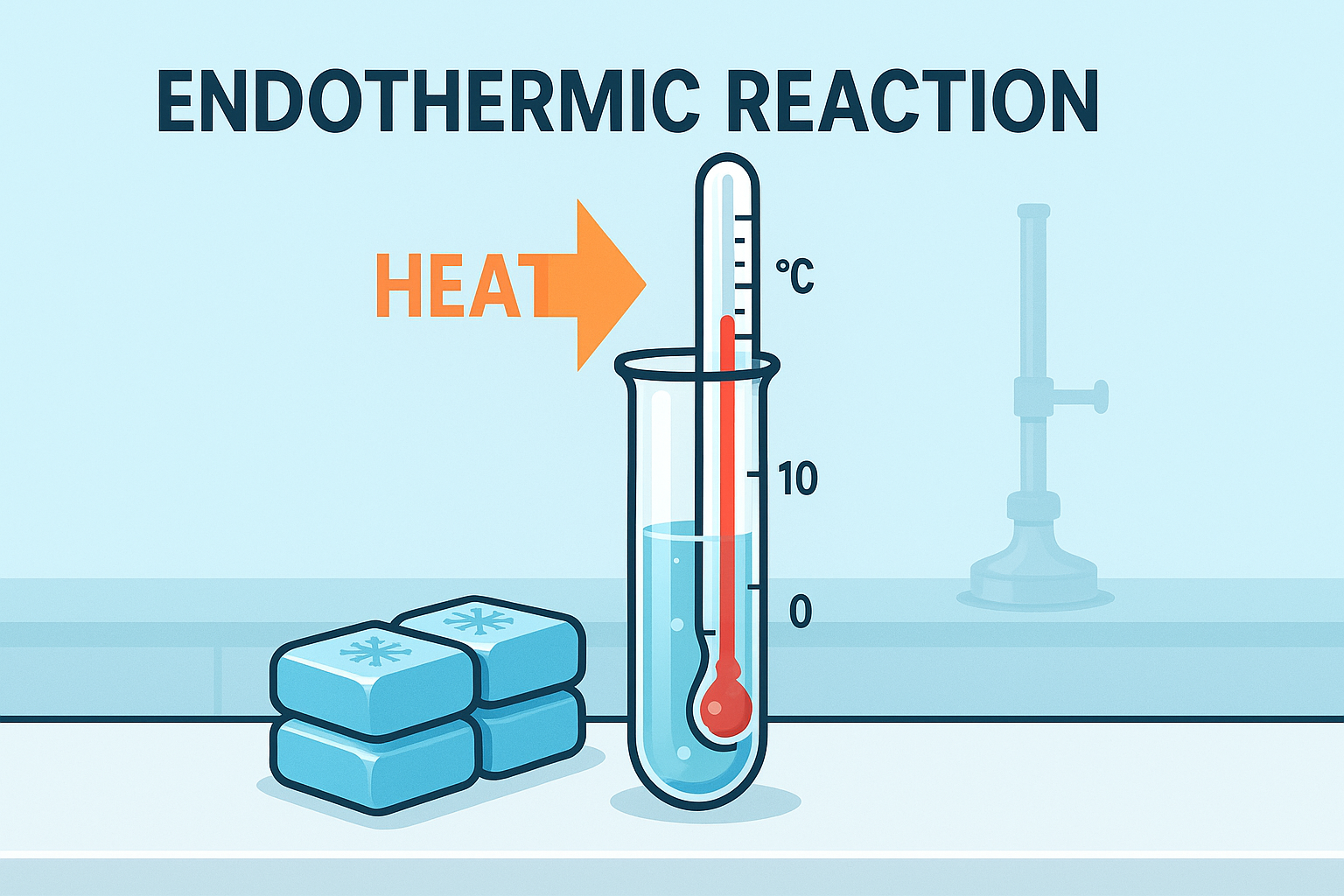
Endothermic Reactions
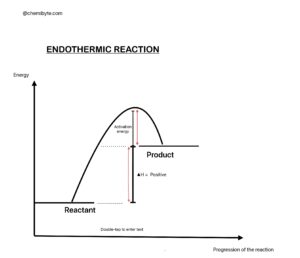
An endothermic reaction absorbs heat energy from the surroundings.
The surroundings become cooler because heat flows into the reaction.
Important meaning:
• The prefix “endo-” means inside or into.
So an endothermic reaction takes heat into the system.
Key features:
• Heat energy is taken in
• Temperature of surroundings decreases
• ΔH is positive because the products have more enthalpy than the reactants
Simple example:
When ammonium nitrate dissolves in water, the temperature drops.
This happens because the reaction absorbs heat from the water.
Energy pattern:
Reactants + energy → Products
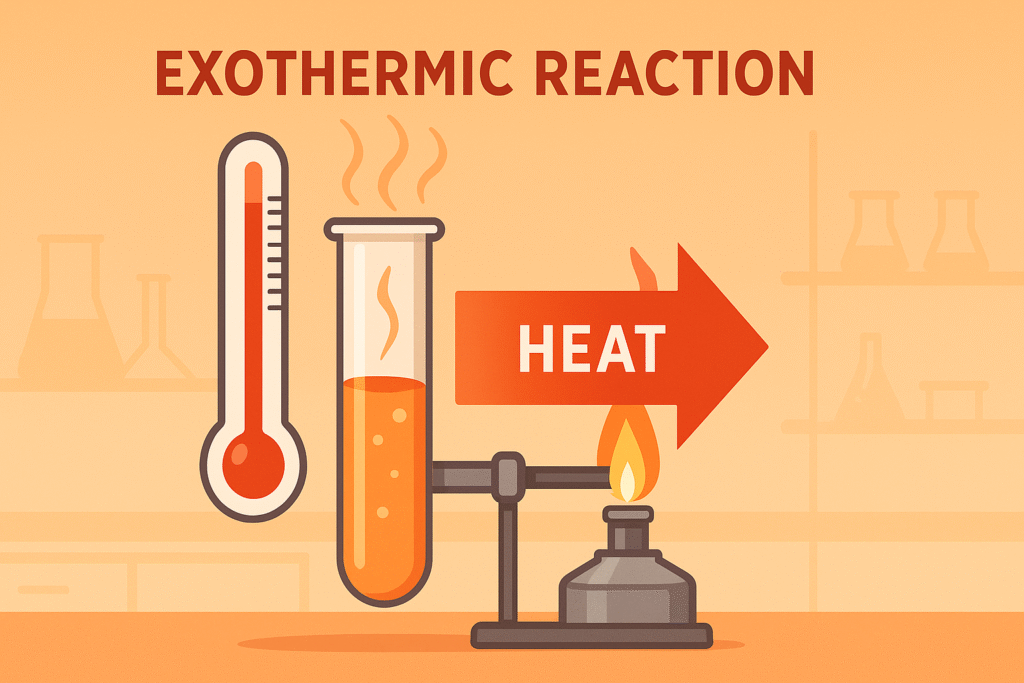
Exothermic Reactions
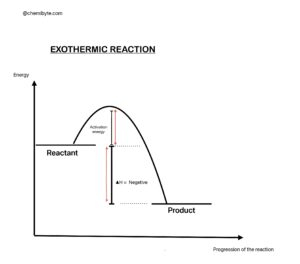
An exothermic reaction releases heat energy to the surroundings.
The surroundings warm up because energy flows out of the reaction.
Important meaning:
• The prefix “exo-” means outside or out of.
So an exothermic reaction gives heat out of the system.
Key features:
• Heat energy is released
• Temperature of surroundings increases
• ΔH is negative because the products have less enthalpy than the reactants
Simple example:
When magnesium reacts with hydrochloric acid, the test tube becomes warm.
Heat is released as new bonds form.
Energy pattern:
Reactants → Products + energy
DIFFERENCE BETWEEN EXOTHERMIC AND ENDOTHERMIC REACTIONS
Comparison Table
| Exothermic Reaction | Endothermic Reaction | |
|---|---|---|
| Meaning | Energy goes out of the reaction (exo = out) | Energy goes into the reaction (endo = into) |
| Heat Transfer | Heat is released to the surroundings | Heat is absorbed from the surroundings |
| Temperature Change | Temperature of surroundings increases | Temperature of surroundings decreases |
| Enthalpy Change (ΔH) | ΔH is negative (products have lower enthalpy) | ΔH is positive (products have higher enthalpy) |
| Energy Diagram | Products are lower than reactants | Products are higher than reactants |
| Energy Equation Pattern | Reactants → Products + energy | Reactants + energy → Products |
| Example Reaction | Burning fuels, magnesium + acid | Dissolving ammonium nitrate, photosynthesis |
Factors That Affect the Enthalpy change in a Reaction
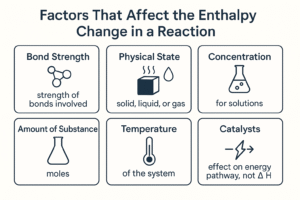
Now that we understand what enthalpy change (ΔH) means and how to identify whether a reaction is exothermic or endothermic, the next step is to look at what affects the amount of energy released or absorbed. Although the basic idea of ΔH remains the same for every reaction, several factors influence how large the energy change will be.
These factors include the strength of bonds involved, the physical state of the substances, and the reaction conditions.
-
Bond Strength
The main factor affecting the size of an energy change is the strength of the bonds that are broken and formed.
-
Strong bonds require more energy to break, increasing the endothermic step.
-
Strong bonds release more energy when they form, increasing the exothermic step.
Example:
In combustion reactions, many strong O–H bonds form in the products (e.g., water). These strong bonds release a large amount of energy, which is why combustion reactions have large negative ΔH values.
-
Physical State of Reactants and Products
The energy change also depends on whether substances are solid, liquid, gas, or aqueous.
-
Gases have higher energy than liquids or solids.
-
Converting a substance from one state to another involves extra energy (latent heat).
Example:
Water produced as steam (gas) contains more energy than water produced as a liquid.
Therefore, the measured enthalpy change differs depending on the state specified.
This is why balanced thermochemical equations always include state symbols (s, l, g, aq).
-
Concentration (for solutions)
In reactions involving solutions, concentration affects the number of available particles that can react.
-
Higher concentration → more collisions → faster reaction.
-
Although ΔH per mole does not usually change, the amount of heat observed in a calorimetry experiment can increase if more reactant particles are actually reacting.
This explains why temperature changes depend on both ΔH and the amount of reactants.
-
Amount of Substance (Moles)
Enthalpy change is always expressed per mole of substance reacting, but in practical experiments:
More moles reacting → more total heat released or absorbed.
Example:
Combusting 2 moles of ethanol releases twice as much heat as combusting 1 mole.
This is why ΔH is standardised to kJ/mol, so reactions can be compared fairly.
-
Temperature of the System
Temperature does not change the fundamental ΔH of a reaction, but it does affect how the reaction proceeds.
-
Higher temperatures give particles more energy.
-
More particles reach or exceed activation energy.
-
Reaction rate increases, but the actual ΔH per mole stays mostly the same.
However, if a process involves dissolving or hydration, slightly different temperature conditions can shift the observed temperature change in a calorimetry experiment.
-
Catalysts (Effect on Energy Pathway, Not ΔH)
Catalysts provide an alternative reaction pathway with a lower activation energy (Ea).
On a reaction profile diagram, this is shown by a smaller peak.
Important point:
A catalyst does not change the enthalpy change, ΔH.
It only makes the reaction happen more easily and quickly.
Why These Factors Matter
Understanding the factors that affect energy changes allows chemists to:
-
Predict how much heat a reaction will release or absorb
-
Compare different fuels or chemical processes
-
Design safer industrial reactions
-
Optimise conditions for maximum efficiency
-
Interpret calorimetry data more accurately
These ideas also help students understand why some reactions feel “hotter” or “colder” even when they involve similar types of processes.
Calculating Enthalpy Change Using Bond Energies
So far, we have looked at energy changes in a qualitative way:
exothermic reactions release heat, and endothermic reactions absorb heat.
To understand energy changes more quantitatively, chemists use bond energies.
Bond energies give us a numerical way to estimate how much energy is taken in or given out during a reaction.
What Are Bond Energies?
Bond energy is the amount of energy needed to break one mole of a specific bond in the gas state (e.g., O–H, C–H, Cl–Cl).
Units: kJ/mol
Important points:
-
Breaking bonds always absorbs energy (endothermic).
-
Forming bonds always releases energy (exothermic).
-
A stronger bond has a higher bond energy.
These values are usually given in tables and are called average bond energies, because actual bond strengths depend on the molecule.
How to Calculate Enthalpy Change Using Bond Energies
Once you understand that chemical reactions involve breaking old bonds and forming new ones, you can begin to calculate the energy change that takes place. Bond energies make this possible. A bond energy tells you how much energy is needed to break one mole of a particular bond in the gas state. Because breaking bonds always absorbs energy and forming bonds always releases energy, comparing the two allows us to estimate the overall enthalpy change, ΔH, for a reaction.
The calculation follows a simple idea: if more energy is needed to break the bonds in the reactants than is released when the new bonds form, the reaction is endothermic and ΔH is positive. If more energy is released when new bonds form than is required to break the old bonds, the reaction is exothermic and ΔH is negative. Bond energy values are usually provided in a data table during exams, and you use these numbers to work out the energy in and the energy out.
The method can be summarised clearly:
• First, add up the energies of all the bonds broken in the reactants.
• Then, add up the energies of all the bonds formed in the products.
• Finally, subtract the second value from the first using the formula:
ΔH = energy in (bonds broken) – energy out (bonds formed).
If the answer is negative, the reaction releases heat. If the answer is positive, the reaction absorbs heat. This approach links directly to the molecular events taking place during a reaction and shows how enthalpy change reflects the balance between bond breaking and bond formation.
Worked Example (Clear, Simple)
Consider the reaction between hydrogen and chlorine to form hydrogen chloride. You are given the following bond energies:
H–H = 436 kJ/mol,
Cl–Cl = 243 kJ/mol,
H–Cl = 431 kJ/mol.
H₂ + Cl₂ → 2HCl
First, find the energy needed to break the bonds in the reactants. One H–H bond and one Cl–Cl bond must be broken,
so the total energy absorbed is 436 + 243 = 679 kJ.
Next, calculate the energy released when the new bonds form. Two H–Cl bonds are made, releasing 2 × 431 = 862 kJ. Now apply the formula:
ΔH = 679 – 862 = –183 kJ.
The negative sign shows that the reaction is exothermic. More energy is released from forming the new H–Cl bonds than is taken in to break the original bonds.
Why Bond Energy Calculations Matter
These calculations allow us to estimate the energy changes in real reactions, compare the effectiveness of different fuels, and understand why some reactions release large amounts of heat while others do not. Although bond energies are averaged values and give approximate results, they provide a reliable method for analysing the energetic behaviour of reactions at the molecular level.
Energy Changes in Different Types of Reactions
Chemical reactions do not all transfer energy in the same way. Some release heat, some absorb heat, and some cause only small temperature changes. The pattern depends on the type of reaction and on the bonds being broken and formed. Below are the main reaction types you need to understand for GCSE and high-school chemistry.
Combustion Reactions
Combustion happens when a substance burns in oxygen. These reactions always give out heat.
• Always exothermic
• Products usually include carbon dioxide and water
• Strong C=O and O–H bonds form, releasing a large amount of energy
• Fuels release a lot of heat because they contain many C–H bonds
Example: burning methane or petrol.
Neutralisation Reactions
Neutralisation happens when an acid reacts with a base or alkali.
• Usually exothermic
• Water forms, and forming O–H bonds releases energy
• Temperature normally rises in the experiment
Example: hydrochloric acid + sodium hydroxide.
Dissolving Reactions
When a substance dissolves in water, the energy change depends on the balance between breaking the structure and hydrating the particles.
• Can be exothermic
• Can be endothermic
• Depends on the substance and its lattice structure
Examples:
• Calcium chloride dissolving → temperature rises
• Ammonium nitrate dissolving → temperature falls
Displacement Reactions
A more reactive metal can replace a less reactive metal from its compound.
• Often exothermic
• Energy is released when stronger bonds form in the product
• The solution may warm up noticeably
Example: zinc placed in copper sulfate solution.
Precipitation Reactions
Two solutions react to form an insoluble solid (a precipitate).
• Usually small energy changes
• Sometimes slightly exothermic
• Depends on the strength of the ionic bonds in the solid formed
Example: silver chloride forming from silver nitrate and sodium chloride.
Redox Reactions
Redox reactions involve transfer of electrons.
• Often involve large energy changes
• Many combustion reactions are redox
• Reactions in batteries rely on redox energy transfers
These reactions can be strongly exothermic or mildly endothermic, depending on the substances involved.
Why These Patterns Are Useful
Knowing the typical energy behaviour of each reaction type helps you:
• Predict temperature changes in experiments
• Understand which reactions release a lot of heat
• Explain cooling and heating effects in real-life situations
• Link reaction type with bond energies and enthalpy change
Energy Changes in Fuels and Food (Comparing Energy Release)
Fuels and foods both store chemical energy in their bonds. When they react, this energy is released and transferred to the surroundings. Although they serve different purposes, the basic idea is the same: the amount of energy released depends on the types of bonds in the molecules and how much energy is released when new bonds form in the products.
Fuels and Their Energy Release
When fuels burn, they undergo combustion. This process releases large amounts of heat.
• Combustion is always exothermic
• Strong C=O and O–H bonds form in the products (CO₂ and H₂O)
• These strong bonds release a lot of energy
• Fuels with many C–H bonds release more energy per gram
Examples: methane, propane, petrol, ethanol.
Why fuels differ in energy:
• Different fuels have different numbers of high-energy bonds
• More C–H bonds → more energy released
• Some fuels burn more completely than others
Measuring the Energy in Fuels
In school experiments, the energy released by a fuel is measured using a simple calorimetry setup.
• Burn a small amount of fuel
• Heat a known mass of water above the flame
• Measure the rise in temperature
• Use the temperature change to calculate energy released
This method is not perfectly accurate because heat escapes to the surroundings, but it allows easy comparison between fuels.
Food as a Source of Chemical Energy
Food stores energy in the bonds of carbohydrates, fats and proteins. When the body respires, these molecules react with oxygen and release energy.
• Respiration is a controlled release of energy
• Fats contain many C–H bonds → high energy content
• Carbohydrates store less energy per gram than fats
School demonstration:
• Burn a small piece of food (e.g., nut or crisp) under a boiling tube of water
• Measure the temperature rise
• Estimate the energy released
Although much heat is lost to the air, the experiment shows that different foods contain different amounts of chemical energy.
Why Comparing Fuels and Foods Matters
Understanding the energy content of fuels and food helps explain:
• Why some fuels are more efficient than others
• How energy is transferred in combustion
• Why high-fat foods provide more energy than high-carbohydrate foods
• How calorimetry links chemical energy to measurable temperature changes
This brings together the ideas of enthalpy change, bond energies and real-world energy transfer.
Reaction Profile Diagrams and Activation Energy
Reaction profile diagrams show how the energy of a system changes as a reaction takes place. They help you visualise the difference between exothermic and endothermic reactions and show the activation energy needed to start the reaction. These diagrams link the molecular events of bond breaking and bond formation to the overall enthalpy change.
What Reaction Profiles Show
A reaction profile diagram includes several key features:
• Energy level of the reactants
• Energy level of the products
• Activation energy (the “hump”)
• The overall enthalpy change, ΔH
These features help you see whether the reaction releases or absorbs energy and how much energy is required to get it started.
Activation Energy
Activation energy is the minimum amount of energy particles need to collide successfully and begin a reaction.
• It appears as the peak on the reaction profile
• A high activation energy means the reaction is harder to start
• A low activation energy means the reaction starts more easily
Before new bonds can form, old bonds must first be broken. Breaking these bonds requires energy, which is why activation energy is always needed.
Exothermic Reaction Profiles
In an exothermic reaction, the products have less energy than the reactants.
• Products are drawn lower on the diagram
• ΔH is negative
• Energy is released to the surroundings
The diagram shows a “drop” from reactants to products, representing the energy released when new bonds form.
Endothermic Reaction Profiles
In an endothermic reaction, the products have more energy than the reactants.
• Products are drawn higher on the diagram
• ΔH is positive
• Energy is absorbed from the surroundings
Here, the diagram shows a “rise,” indicating that the system takes in energy overall.
How Catalysts Affect Reaction Profiles
A catalyst provides an alternative reaction pathway with a lower activation energy.
• The peak on the diagram becomes lower
• The reaction starts more easily
• ΔH does not change
Catalysts do not alter the reactants or products; they only change the pathway, so the enthalpy change stays the same.
Why Reaction Profiles Matter
Reaction profile diagrams help students:
• Identify exothermic and endothermic reactions
• Understand activation energy
• Compare reactions with and without catalysts
• Link molecular processes to measurable energy changes
They are a central tool in learning how energy moves during chemical reactions.
Energy Changes in Reversible Reactions
Reversible reactions are reactions that can go in both directions. In one direction, the reactants form products; in the reverse direction, the products change back into the original reactants. What makes reversible reactions interesting is that the energy change in one direction is always the opposite of the energy change in the other direction.
Energy in the Forward and Reverse Directions
A reversible reaction cannot be exothermic in both directions or endothermic in both directions. If the forward reaction releases energy, then the reverse reaction must absorb the same amount of energy. If the forward reaction absorbs energy, the reverse reaction must release that exact amount back.
Key idea:
• Forward exothermic → reverse endothermic
• Forward endothermic → reverse exothermic
The size of the energy change is the same in both directions, but the sign changes.
Example of a Reversible Reaction
One common example is the decomposition and formation of hydrated copper sulfate.
CuSO₄·5H₂O (blue) ⇌ CuSO₄ (white) + 5H₂O
• Heating the blue crystals drives off the water molecules.
• This process is endothermic because heat is absorbed.
• Adding water back to the white anhydrous solid reforms the blue crystals.
• This reverse reaction is exothermic because heat is released.
The pair of reactions demonstrates the “mirror image” energy changes that reversible reactions always show.
Energy Profile for a Reversible Reaction
A reversible reaction can be shown on a diagram with two energy pathways: one for the forward reaction and one for the reverse.
• If the forward direction is exothermic, the energy level of the products is lower than the reactants.
• The reverse direction will then require energy input, showing an upward change in energy.
• Both directions have their own activation energies, which may be different.
The diagram makes it clear how energy flows depending on the direction of the reaction.
Why Energy in Reversible Reactions Matters
Understanding the energy pattern in reversible reactions helps you:
• Predict whether heating or cooling will push a reaction forward or backward
• Explain observations such as colour changes or temperature changes
• Connect energy ideas to equilibrium (a later GCSE/High-school topic)
• Understand how industry controls reactions to maximise yield
Reversible reactions show that energy changes are not random: they follow strict rules that connect directly to the direction of the reaction.
Bringing Everything Together: Summary of Energy Changes in Chemistry
Energy changes are at the heart of every chemical reaction. Whether a reaction releases heat, absorbs heat, changes temperature only slightly or dramatically, or shifts direction depending on conditions, all of these behaviours come from the same core idea: bonds must be broken and formed, and energy is transferred in the process. Understanding these energy transfers gives meaning to reaction patterns, practical experiments and real-world applications.
Throughout this topic, you learned how molecules store energy and why chemical reactions always involve energy changes. You explored the meaning of enthalpy and enthalpy change, and how ΔH tells us whether a reaction is exothermic or endothermic. You also learned how to measure these changes using calorimetry and how to calculate them using bond energies. Reaction profile diagrams helped you visualise activation energy and catalyst effects, while reversible reactions showed you how energy behaves when reactions can go in both directions.
To complete your understanding, here are the most important ideas to remember:
• Breaking bonds absorbs energy; forming bonds releases energy.
• Exothermic reactions release heat and have negative ΔH.
• Endothermic reactions absorb heat and have positive ΔH.
• Calorimetry allows us to measure temperature change and estimate energy transfer.
• Bond energy calculations compare energy in (breaking bonds) and energy out (forming bonds).
• Reaction profiles show activation energy and whether ΔH is positive or negative.
• Catalysts lower the activation energy but do not change ΔH.
• In reversible reactions, one direction is exothermic and the other is endothermic.
These ideas link together to form a complete picture of how energy moves in chemistry. They explain everyday processes such as burning fuels, dissolving salts, using cold packs or hand warmers and the way the body releases energy from food. They also provide the foundation for more advanced topics like equilibrium, rates of reaction and energetics in industrial chemistry.
Understanding energy changes helps you think like a chemist. It allows you to interpret experimental results, explain temperature changes, compare different substances and predict how a reaction will behave. With these concepts mastered, you are ready to move on to the next stage of chemistry with confidence.
Read more