Introduction: Acids, Bases and Alkalis
What is the first thing that comes to your mind when you think about acids and bases?
Most people think about the acid in their stomach or the citric acid in lemons when they hear the word acid. When they think about bases, they often remember toothpaste or soap, which are used every day at home.
Although acids and bases might sound complicated, they are actually very familiar substances that we encounter in daily life. These chemicals are responsible for many important reactions, from helping us digest food to keeping our teeth healthy and cleaning our hands.
In GCSE Chemistry, acids, bases and alkalis form a key topic that helps students understand pH, neutralisation reactions, indicators, and the preparation of salts. Learning these ideas clearly at the beginning makes it much easier to succeed in exams and practical work later on.
What Are Acids, Bases and Alkalis? (GCSE)
What Is an Acid?
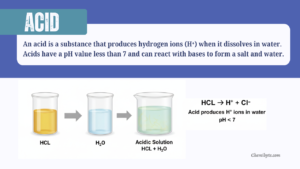
An acid is a substance that produces hydrogen ions (H⁺) when it dissolves in water.
Acids have a pH value less than 7 and can react with bases to form a salt and water.
Common examples of acids include:
-
Hydrochloric acid (found in the stomach)
-
Citric acid (found in lemons and oranges)
-
Ethanoic acid (found in vinegar)
Acids – Producing Hydrogen Ions
Hydrochloric acid in water:
HCl(aq) → H⁺(aq) + Cl⁻(aq)
Nitric acid in water:
HNO₃(aq) → H⁺(aq) + NO₃⁻(aq)
Sulfuric acid in water:
H₂SO₄(aq) → 2H⁺(aq) + SO₄²⁻(aq)
These equations show that acids release hydrogen ions (H⁺) when dissolved in water.
What Is a Base and an Alkali?
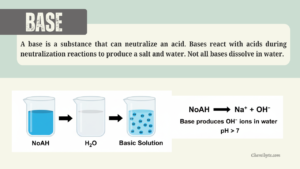
An alkali is a base that dissolves in water and produces hydroxide ions (OH⁻).
Alkalis have a pH value greater than 7.
Common examples of alkalis include:
-
Sodium hydroxide
-
Potassium hydroxide
-
Soap
-
Toothpaste
Bases / Alkalis – Producing Hydroxide Ions
Sodium hydroxide in water:
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
Potassium hydroxide in water:
KOH(aq) → K⁺(aq) + OH⁻(aq)
These equations show that alkalis produce hydroxide ions (OH⁻) in solution.
Summary of Differences in between Acid and Bases
-
Acid: Produces hydrogen ions (H⁺)
-
Base: Neutralizes acids
-
Alkali: A soluble base that produces hydroxide ions (OH⁻)
Understanding these definitions is essential for learning about the pH scale, indicators, and neutralization reactions in GCSE Chemistry.
The pH Scale Explained (GCSE)
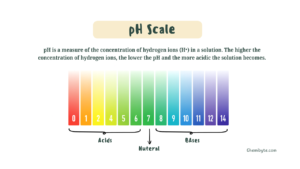
The pH scale is used to measure how acidic or alkaline a solution is.
pH is a measure of the concentration of hydrogen ions (H⁺) in a solution. The higher the concentration of hydrogen ions, the lower the pH and the more acidic the solution becomes.
You may know that some substances are acids or bases by name, but this is not always helpful. Many substances do not have obvious names that tell us whether they are acidic or alkaline. Because of this, scientists needed a simple way to compare how acidic or alkaline different substances are. This is why the pH scale was introduced. It allows us to identify whether a substance is an acid, a base, or neutral, and to compare their strength.
pH is a measure of the concentration of hydrogen ions (H⁺) in a solution. The higher the concentration of hydrogen ions, the lower the pH and the more acidic the solution is.
The pH scale runs from 0 to 14:
-
pH less than 7 → acidic
-
pH equal to 7 → neutral
-
pH greater than 7 → alkaline
A solution with a low pH is strongly acidic, while a solution with a high pH is strongly alkaline.
Why pH 7 Is Neutral
A solution is neutral when it has equal concentrations of hydrogen ions (H⁺) and hydroxide ions (OH⁻).
Pure water has a pH of 7, which is why it is described as neutral.
Strong and Weak Acids (GCSE O/ Level)
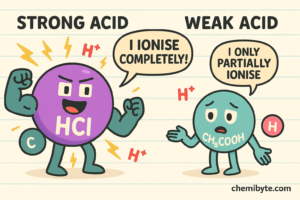
A strong acid is an acid that completely ionises in water, meaning it releases a large number of hydrogen ions (H⁺).
An example of a strong acid is hydrochloric acid.
A weak acid is an acid that partially ionises in water, meaning it releases fewer hydrogen ions (H⁺).
An example of a weak acid is ethanoic acid.
In simple terms, a strong acid is stronger because it produces more hydrogen ions, not because there is more acid present. A weak acid produces fewer hydrogen ions, even if the same amount of acid is dissolved in water.
At GCSE level, it is very important to remember that strength is not the same as concentration.
-
Strength depends on how much the acid ionises.
-
Concentration depends on how much acid is dissolved in water.
Strong Acid – Equation Example
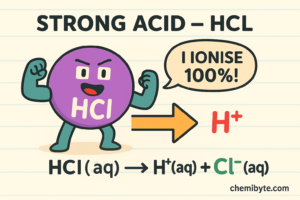
A strong acid completely ionises in water.
Hydrochloric acid in water: HCl(aq) → H⁺(aq) + Cl⁻(aq)
This shows that hydrochloric acid releases all of its hydrogen ions, which is why it is a strong acid.
Weak Acid – Equation Example
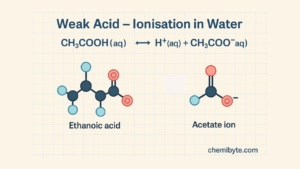
A weak acid only partially ionises in water.
Ethanoic acid in water:
CH₃COOH(aq) ⇌ H⁺(aq) + CH₃COO⁻(aq)
The double arrow (⇌) shows that only some of the acid molecules ionise, which is why ethanoic acid is a weak acid.
GCSE Exam Tip
-
Single arrow (→) = strong acid (complete ionisation)
-
Double arrow (⇌) = weak acid (partial ionisation)
Strong and Weak Bases (GCSE / O-Level)
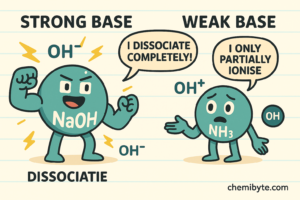
A strong base is a base that completely ionises in water, meaning it releases a large number of hydroxide ions (OH⁻).
An example of a strong base is sodium hydroxide.
A weak base is a base that partially ionises in water, meaning it releases fewer hydroxide ions (OH⁻).
An example of a weak base is ammonia solution.
In simple terms, a strong base is stronger because it produces more hydroxide ions, not because there is more base present. A weak base produces fewer hydroxide ions, even if the same amount of base is dissolved in water.
At GCSE level, it is very important to remember that strength is not the same as concentration.
-
Strength depends on how much the base ionises.
-
Concentration depends on how much base is dissolved in water.
Strong Base – Equation Example
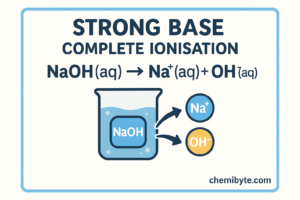
A strong base completely ionises in water.
Sodium hydroxide in water:
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
This shows that sodium hydroxide releases all of its hydroxide ions, which is why it is a strong base.
Weak Base – Equation Example
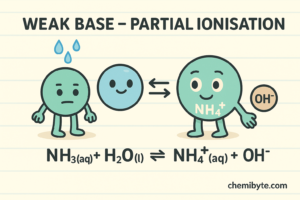
A weak base only partially ionises in water.
Ammonia in water:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
The double arrow (⇌) shows that only some of the base molecules ionise, which is why ammonia is a weak base.
GCSE Exam Tip
-
Single arrow (→) = strong base (complete ionisation)
-
Double arrow (⇌) = weak base (partial ionisation)
Indicators and pH Measurement (GCSE)
Indicators are substances that change colour depending on the pH of a solution.
Often in the laboratory, we are given a solution without knowing what it is. The solution may be colourless and have no label, so we cannot tell by looking at it whether it is an acid, a base, or neutral. Tasting or touching chemicals is unsafe, so we need a safe and reliable method to identify them.
This is where indicators are used. By adding a few drops of an indicator or dipping indicator paper into the solution, the colour change tells us whether the solution is acidic, neutral, or alkaline. In this way, indicators help us identify the nature of unknown solutions quickly and safely.
Litmus Paper as and Indicator
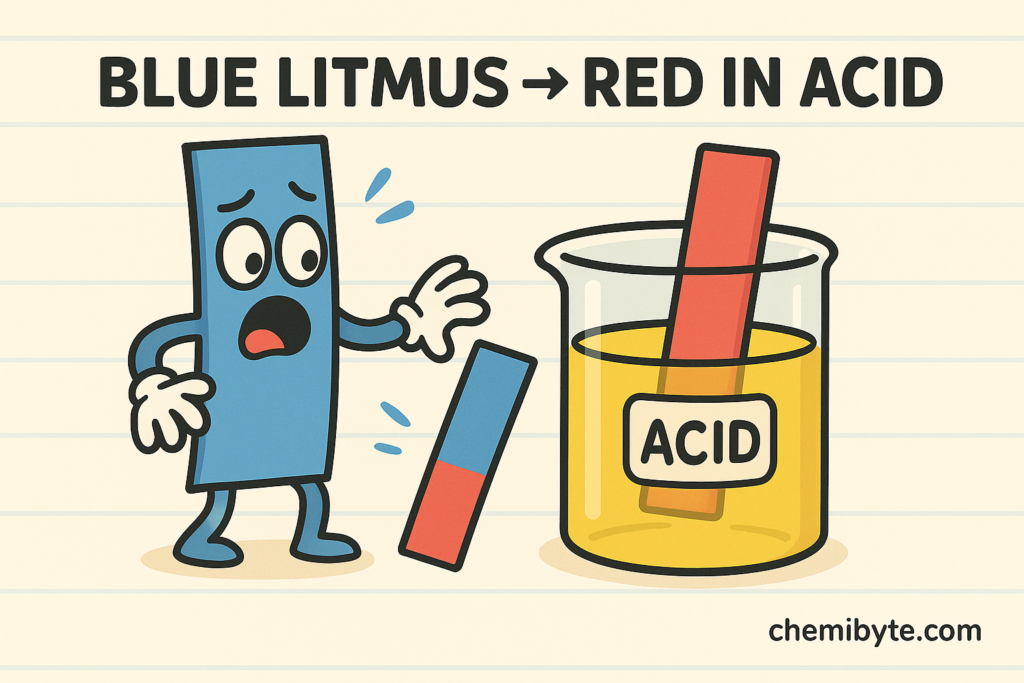
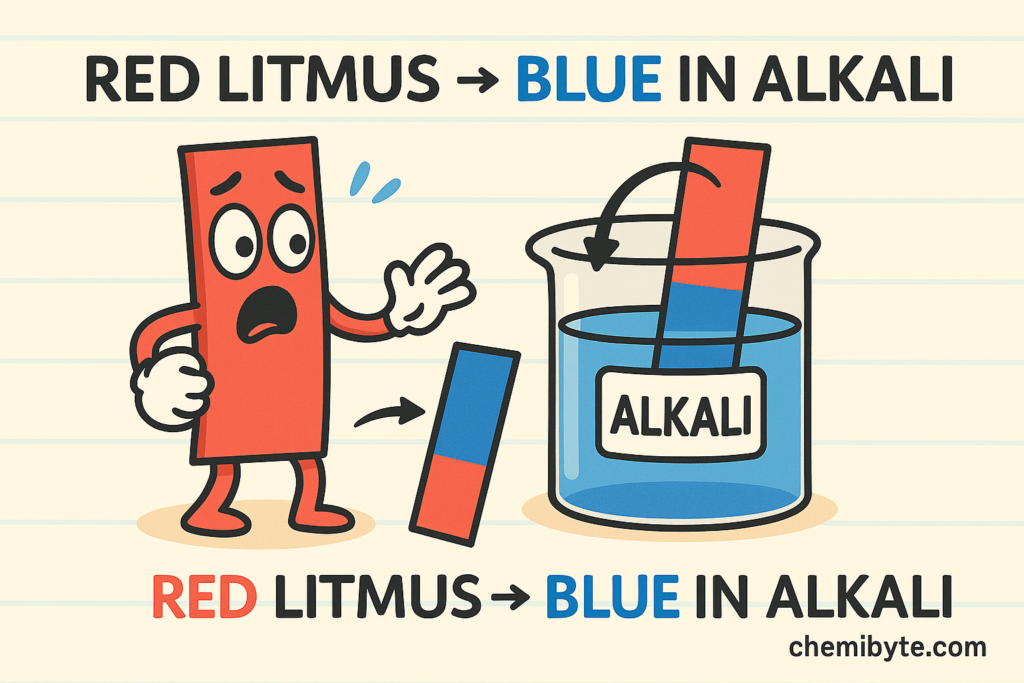
Litmus paper is one of the simplest indicators used in school laboratories. It comes in two forms: red litmus paper and blue litmus paper.
-
Blue litmus paper turns red in acidic solutions
-
Red litmus paper turns blue in alkaline solutions
Litmus paper is useful when we only need a quick answer to find out whether a solution is an acid or an alkali. However, it has a limitation: it cannot show how strong the acid or alkali is and does not provide a pH value. It only gives a yes or no result.
Universal Indicator

Universal indicator is more advanced than litmus paper. It is a mixture of different indicators, which allows it to show many different colours across the pH scale.
Each colour corresponds to a specific pH range:
-
Red shows strong acids
-
Yellow to orange shows weak acids
-
Green shows neutral solutions
-
Blue to purple shows alkalis
Because of this, universal indicator is very useful when we want to estimate the pH of a solution rather than just identify it as acidic or alkaline. This makes it especially helpful in GCSE practical experiments.
Phenolphthalein as a indicator
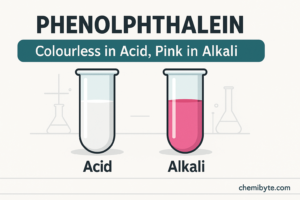
Phenolphthalein is another commonly used indicator, especially in titration experiments.
-
It is colourless in acidic solutions
-
It turns pink in alkaline solutions
Phenolphthalein is useful because its colour change is very clear and sudden, making it easy to see the exact point at which a solution changes from acidic to alkaline. At GCSE level, you only need to remember its colour change, not the detailed chemistry behind it.
pH Meter as and Indicator
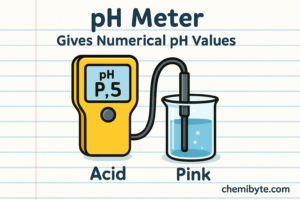
A pH meter is an electronic device that measures pH directly. Instead of showing a colour, it gives a numerical pH value, such as 3.5 or 9.2.
pH meters are:
-
More accurate than indicators
-
Used when precise results are needed
-
Commonly used in laboratories, industry, and environmental testing
Why Indicators Are Important
Indicators and pH meters are important because they allow chemists to:
-
Identify unknown solutions safely
-
Compare how acidic or alkaline different substances are
-
Carry out neutralisation reactions accurately
-
Perform required practicals correctly in GCSE exams
Understanding how indicators work helps students answer exam questions clearly and perform practical experiments confidently.
Neutralization reactions explained (Step by Step)
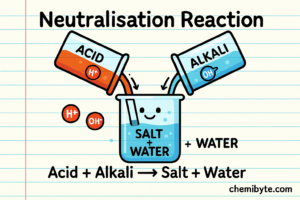
When an acid is dissolved in water, it breaks up to produce hydrogen ions (H⁺).
These hydrogen ions are the reason a solution is acidic.
When an alkali is dissolved in water, it produces hydroxide ions (OH⁻).
These hydroxide ions are the reason a solution is alkaline.
So:
-
H⁺ ions cause acidity
-
OH⁻ ions cause alkalinity
What Happens When an Acid and an Alkali Are Mixed
When an acid and an alkali are mixed together, their ions move freely in the solution.
The hydrogen ions (H⁺) from the acid are attracted to the hydroxide ions (OH⁻) from the alkali.
When they meet, they react together and form water (H₂O).
This can be written as the ionic equation: H⁺(aq) + OH⁻(aq) → H₂O(l)
Why This Reaction Is Called Neutralisation
Before the reaction:
-
The solution is acidic because of H⁺ ions
-
Or alkaline because of OH⁻ ions
After the reaction:
-
The H⁺ ions are used up
-
The OH⁻ ions are used up
-
They are turned into neutral water
Because the ions that cause acidity and alkalinity are removed, the solution becomes neutral or closer to neutral. This is why the reaction is called neutralization.
Why Other Ions Are Not Shown
In reactions like: HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
The sodium ions (Na⁺) and chloride ions (Cl⁻) do not change. They stay in solution and form a salt. These ions are called spectator ions, so they are not included in the ionic equation.
The ionic equation only shows the important chemical change, which is: H⁺(aq) + OH⁻(aq) → H₂O(l)
GCSE Exam Key Idea
All neutralisation reactions are based on the same process: hydrogen ions reacting with hydroxide ions to form water.
If you understand this one ionic equation, you can understand every neutralisation reaction at GCSE level.
Salts are formed when an acid reacts with another substance such as a metal, a base, or a carbonate. In all of these reactions, the hydrogen ions (H⁺) from the acid are replaced by metal ions, which leads to the formation of a salt.
However, not all substances behave in the same way in water. Some substances dissolve in water (soluble), while others do not dissolve (insoluble). Because of this, the method used to prepare a salt must be chosen carefully.
If a reactant is soluble, it can easily mix with the acid, but it may be difficult to stop the reaction at the correct point. If a reactant is insoluble, it can be added in excess and any unreacted solid can be removed by filtration, making the process easier and safer to control.
This is why chemists choose different methods to prepare salts depending on:
-
the type of reactants used (metal, base, or carbonate)
-
whether the reactants are soluble or insoluble in water
-
how easily the excess reactant can be removed
Choosing the correct method ensures that the salt is produced safely, purely, and efficiently, which is especially important in GCSE practical work and exam questions.
Preparation of Salts (GCSE / O-Level)
Salts are made when an acid reacts with another substance such as a metal element, a base, or a carbonate. In these reactions, the hydrogen ions (H⁺) in the acid are replaced by metal ions, forming a salt.
Different salts are prepared in different ways because not all substances behave the same during reactions. Some reactants dissolve in water, while others do not. Some reactions release gases, while others do not. Because of these differences, one method may be safe and easy to control for one salt but unsuitable for another. Choosing the correct method helps ensure that the reaction goes to completion and that no unwanted reactants remain in the final product.
We also prepare salts because many salts are useful substances. Salts are used in fertilisers, medicines, food preservation, cleaning products, and many industrial processes. In the laboratory, preparing salts allows chemists to obtain them in a pure form so their properties can be studied or used safely.
Using the correct method helps produce a pure salt and makes the reaction easier to control, which is especially important in GCSE practical work and exam questions.
1.Acid + Metal Element
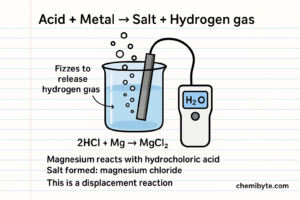
When an acid reacts with a metal element, a displacement reaction takes place (this means a more reactive element replaces a less reactive one in a compound). The metal is more reactive than hydrogen, so it removes hydrogen from the acid.
Acids contain hydrogen ions (H⁺) in solution. During the reaction, the metal atoms lose electrons and form metal ions, while the hydrogen ions gain electrons and form hydrogen gas (H₂). This hydrogen gas escapes from the solution, which causes fizzing.
After the hydrogen is removed, the remaining part of the acid combines with the metal ions to form a salt.
Key points to remember
-
This reaction only happens if the metal is above hydrogen in the reactivity series
-
Hydrogen gas is always produced
-
The products are always a salt and hydrogen
Example Reaction
Word equation:
Magnesium + hydrochloric acid → magnesium chloride + hydrogen
Symbol equation (WordPress-safe): Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
In this reaction:
-
magnesium replaces hydrogen in the acid
-
magnesium chloride is the salt formed
-
hydrogen gas is released, causing fizzing
GCSE exam tip : If you see acid + metal element, always write:salt + hydrogen as the products.
2.Acid + Metal Oxide or Metal Hydroxide
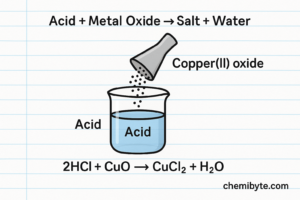
Metal oxides and metal hydroxides are bases. When they react with an acid, a neutralisation reaction takes place. In this type of reaction, the hydrogen ions from the acid react with oxide ions or hydroxide ions from the base to form water.
Because water is formed, these reactions do not produce hydrogen gas. Instead, the products are always a salt and water.
Why this method is commonly used
Many metal oxides and metal hydroxides are insoluble in water. This makes the reaction easier to control because excess solid can be added until all the acid has reacted. Any unreacted solid can then be removed by filtration, leaving a pure salt solution.
Example Reaction
Word equation: Copper oxide + sulfuric acid → copper sulfate + water
Symbol equation : CuO(s) + H2SO4(aq) → CuSO4(aq) + H2O(l)
In this reaction:
-
copper oxide acts as the base
-
sulfuric acid provides hydrogen ions
-
copper sulfate is the salt formed
-
water is produced during neutralisation
Key points to remember
-
Acid + metal oxide / hydroxide → salt + water
-
This is a neutralisation reaction
-
No gas is produced
-
Insoluble bases are easy to remove by filtration
3.Acid + Carbonate
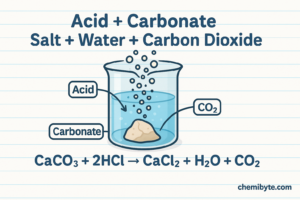
Carbonates react with acids in a different way compared to metals and metal oxides. When an acid reacts with a carbonate, three products are always formed: a salt, water, and carbon dioxide gas. This happens because the carbonate ion reacts with hydrogen ions from the acid, producing carbon dioxide. The gas then escapes from the solution, which makes this reaction easy to recognise.
What you observe in the reaction
-
Fizzing or bubbling occurs
-
The fizzing is caused by carbon dioxide gas being released
Example Reaction
Word equation: Calcium carbonate + hydrochloric acid → calcium chloride + water + carbon dioxide
Symbol equation : CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
In this reaction:
-
calcium carbonate is the carbonate
-
hydrochloric acid reacts with it
-
calcium chloride is the salt formed
-
water and carbon dioxide are also produced
Key points to remember
-
Acid + carbonate → salt + water + carbon dioxide
-
Carbon dioxide gas causes fizzing
-
This reaction is common in laboratory experiments and GCSE exams
Choosing the Correct Method for Preparing Salts (GCSE / O-Level)
Different salts must be prepared using different methods because reactants behave differently in reactions. Some substances dissolve in water, while others do not. Because of this, one method may work well for one salt but not for another. The correct method for preparing a salt depends mainly on what the acid is reacting with and whether that substance is soluble or insoluble in water. Using the correct method makes sure that:
-
all the acid reacts properly
-
no unwanted reactants remain in the final solution
-
the salt produced is pure
For example, insoluble bases and carbonates can be added in excess and then removed by filtration, while soluble bases (alkalis) cannot be filtered and therefore require a titration method. Metal elements must also be chosen carefully so that they are above hydrogen in the reactivity series.
Choosing the correct method is essential for GCSE practical experiments and is a common focus in exam questions.
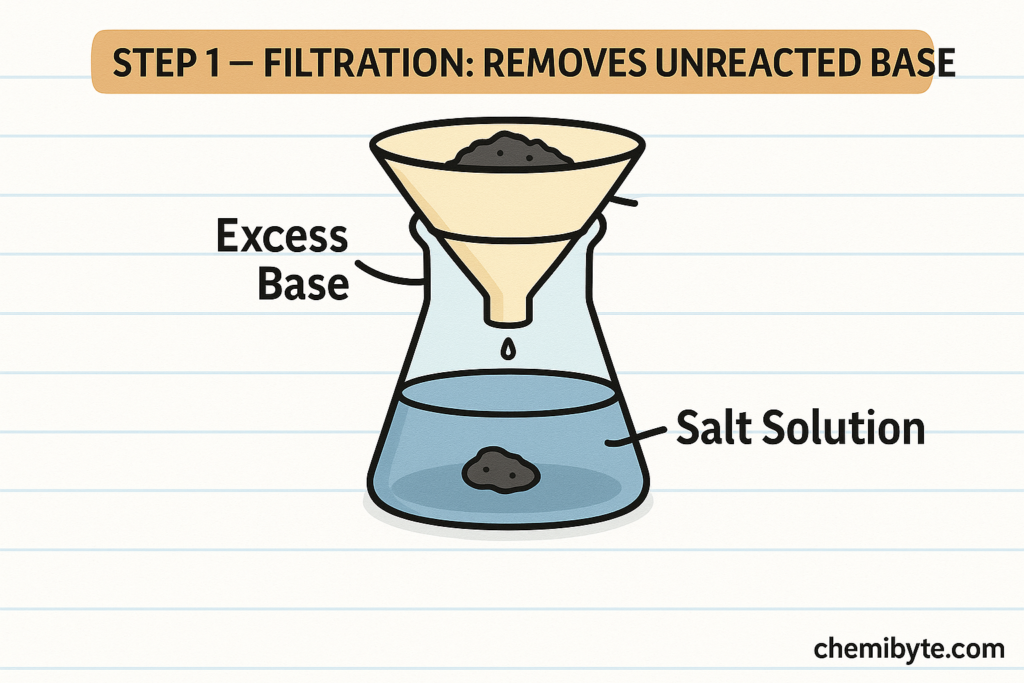
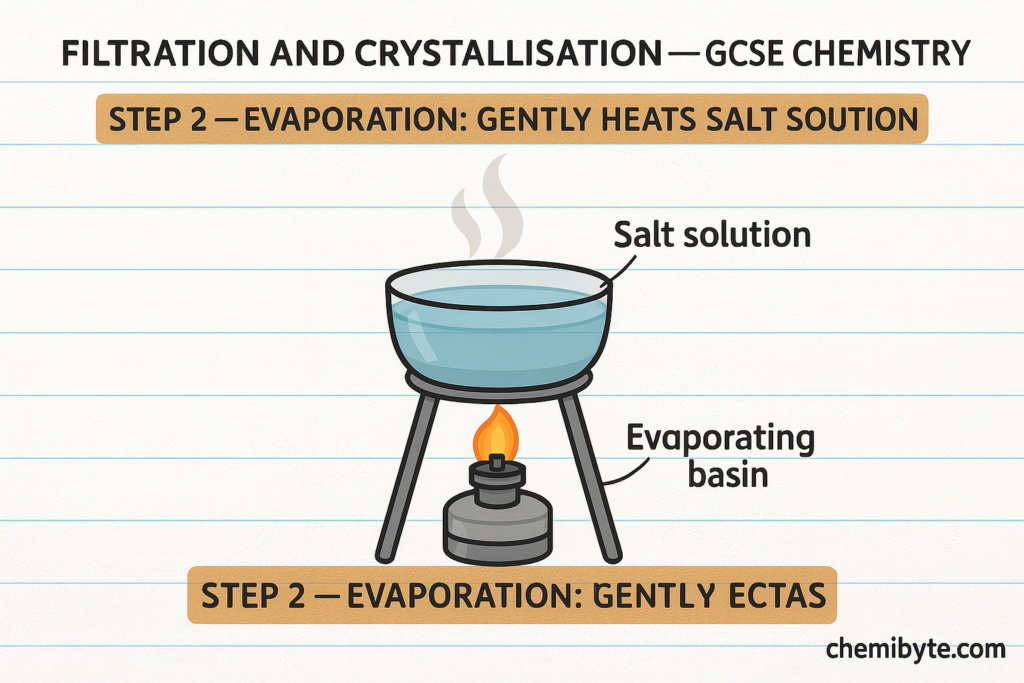
Filtration and Crystallisation (GCSE Chemistry)
After the salt-forming reaction between the acid and the metal, base, or carbonate has fully finished, any excess solid is removed by filtration. This step is used only when an insoluble solid has been added in excess to make sure all the acid has re
acted. Filtration separates the unreacted solid from the liquid, leaving a solution that contains only the dissolved salt and water.
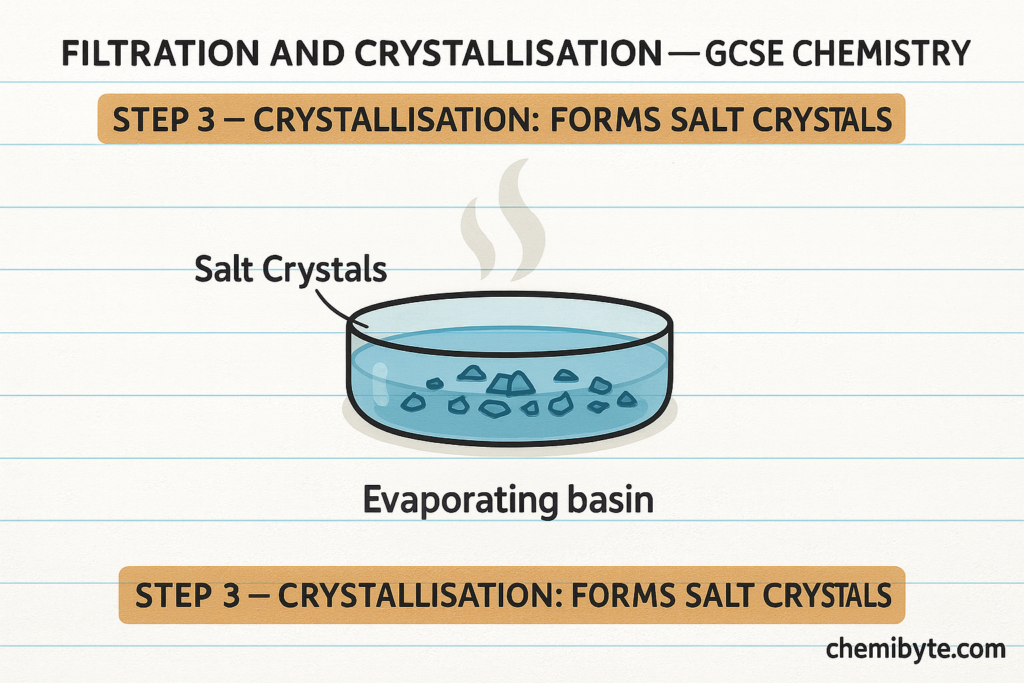
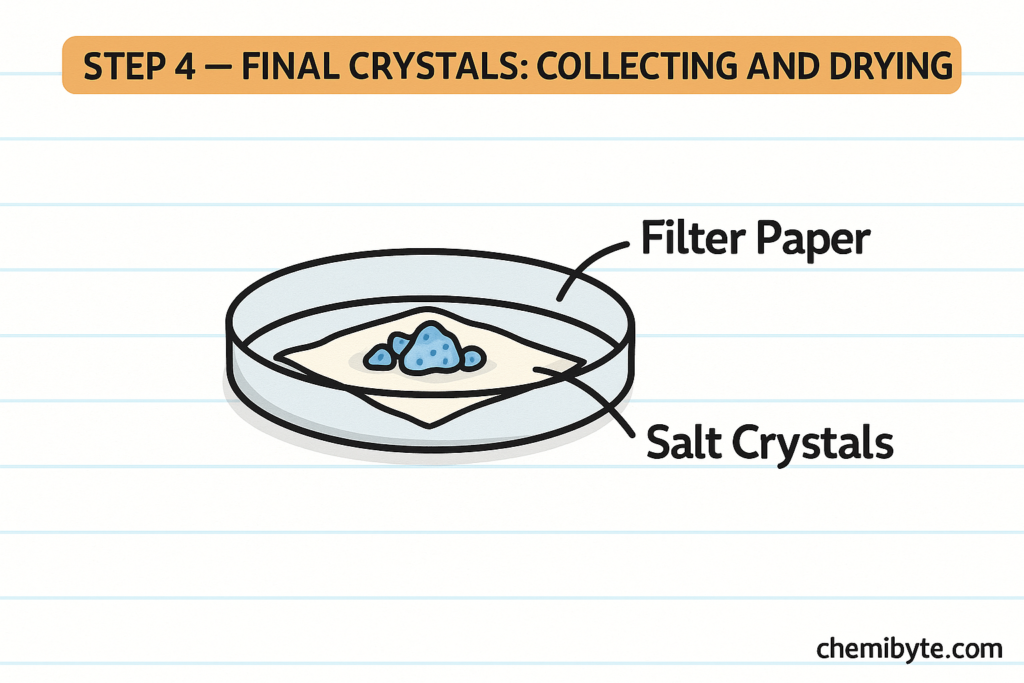
The salt solution is then gently heated so that some of the water evaporates. As the water evaporates, the solution becomes more concentrated. When the solution is allowed to cool, solid salt crystals begin to form. This process is called crystallisation.
Crystallisation is used instead of complete evaporation because it produces larger, well-formed crystals rather than a dry powder. These techniques are important practical skills in GCSE Chemistry experiments and are commonly assessed in exam questions.
Common GCSE Exam Mistakes (Acids, Bases and Salts)
Mistake 1: Confusing Strong and Concentrated Acids (GCSE Chemistry)
Mistake 2: GCSE Chemistry pH Scale Mistakes Students Make
Mistake 3: Writing Incorrect or Unbalanced Chemical Equations (GCSE Chemistry)
Mistake 4: Missing Key Scientific Keywords in GCSE Chemistry Answers
Mistake 5: Choosing the Wrong Method for Preparing Salts (GCSE Chemistry)