Everything around us is made of atoms – but what is an atom, really?
From the screen you’re reading this on to the water you drink, atoms are everywhere. They’re the basic building blocks of all matter — but many students find the idea of atoms a bit confusing at first.
This blog is written just for you — a simple, clear guide to help you understand atoms, step by step.
In this post, you’ll learn:
-
What an atom actually is
-
What parts make up an atom
-
How scientists discovered the structure of atoms over time
Let’s break it down together — nice and easy.
What Is an Atom?
Think about this: you’re crushing a piece of gold.
First, you break it into two parts. Then into smaller and smaller bits. But no matter how many times you break it, it’s still gold — it doesn’t lose its identity. It looks smaller, but it’s still the same substance.
Now here’s the question: how small can you really go?
Even if you break it until you can’t see it anymore, is there a limit?
Scientists asked the same thing. They went far beyond what our eyes can see — and discovered something amazing.
They found that all substances around us — gold, air, water, wood — are made from tiny building blocks called atoms.
These atoms are so small that you can’t see them without special equipment. But they are the basic units of matter — meaning everything you see, touch, or feel is made of atoms.
What Are the Parts of an Atom?
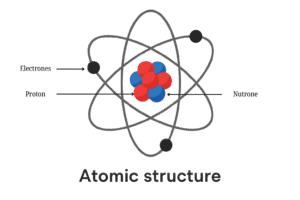
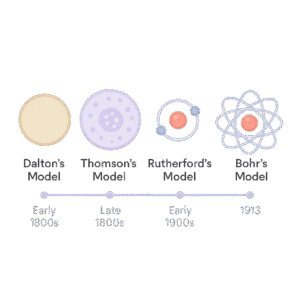
At first, scientists had no idea what atoms looked like. Technology wasn’t advanced back then, so they couldn’t see atoms directly. Instead, they used different experiments and guessed what atoms might look like.
Because of that, many different atomic models were suggested over time. Some were just ideas. Others were based on results from real experiments.
Today, scientists understand atoms much better but in GCSE and school-level chemistry, we often use the Dalton Model or the basic Bohr Model to explain atomic structure.
 According to Bohr’s atomic model, an atom has a tiny central nucleus, and electrons move around it in fixed shells or paths just like planets around the sun (but much, much smaller).
According to Bohr’s atomic model, an atom has a tiny central nucleus, and electrons move around it in fixed shells or paths just like planets around the sun (but much, much smaller).
At first, scientists believed that the atom was the smallest unit of matter, that it couldn’t be divided any further. But later, with better experiments and technology, they discovered that atoms are actually made of even smaller particles.
These smaller parts are called subatomic particles, and there are three main ones you need to know:
-
Proton
-
Neutron
-
Electron
Each one has a different charge, mass, and place inside the atom and they all work together to form the structure of an atom.
Back in the day, scientists couldn’t see atoms and definitely not what was inside them. They had no microscopes strong enough. So how did they find out that atoms are made of smaller particles?
They didn’t just guess. Instead, they did clever experiments and looked at how atoms behaved. By studying electricity, radiation, and how particles moved, they slowly discovered the proton, neutron, and electron.
So, What Makes These Sub Atomic Particles Different?
Let’s break them down simply:
-
Some have positive charge, some have none, and some are negative
-
Some have mass, some are lightweight
-
And they don’t all live in the same part of the atom
Here’s a clear table to help you understand:
Subatomic Particles Summary Table
| Particle | Charge | Mass (Relative) | Where It’s Found | What It Does |
|---|---|---|---|---|
| Proton | +1 (Positive) | 1 | In the nucleus (center) | Decides the element (atomic number) |
| Neutron | 0 (No charge) | 1 | In the nucleus (with protons) | Adds mass, keeps nucleus stable |
| Electron | –1 (Negative) | ~0 (very small) | In shells orbiting the nucleus | Involved in bonding and reactions |
How Do Subatomic Particles Behave Inside the Atom?
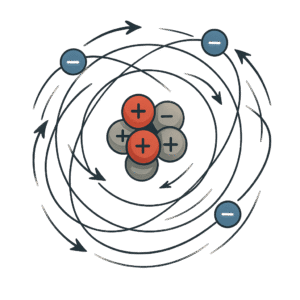
So far, we’ve looked at what the subatomic particles are but now let’s talk about how they behave.
According to Bohr’s Model, the atom isn’t just a still picture. Inside the atom, things are constantly moving. Especially the electrons.
Electrons:
-
Electrons don’t just sit anywhere, they move in fixed paths called energy levels or shells
-
Each shell has a specific energy , the closer to the nucleus, the lower the energy
-
Electrons in outer shells have more energy
-
They move extremely fast so fast that we can’t know exactly where they are at any moment, but we know the area where they’re likely to be
You can think of them like tiny planets zooming around the sun (the nucleus), but much faster and with their own energy zones
Protons and Neutrons:
-
They stay in the nucleus, packed tightly together
-
They don’t move around like electrons
-
Their main job is to give mass and identity to the atom, and keep it stable
Protons and neutrons stay packed together in the center of the atom, that’s the nucleus.
Electrons are much smaller and move around the nucleus in shells or energy levels.
So when we take a closer look at an atom, we can notice that it has some important characteristics things like:
-
Atomic number
-
Mass number
-
And whether the atom has a charge or not
These help us identify the atom and understand how it behaves in chemical reactions.
Let’s break each one down clearly so it’s easy to understand.
Atomic Number and Mass Number
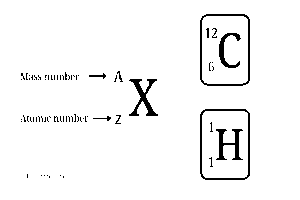
When we look at an atom, two of the most important things we can find are its atomic number and mass number. These numbers help us understand what the atom is, how heavy it is, and how it might react with other atoms.
Atomic Number – The Atom’s Identity Card (A)
The atomic number is a constant for each element. That means it never changes. The atomic number acts like an identification number for every atom. It’s unique no two different elements can have the same atomic number.
We can identify any atom just by knowing its atomic number, because it tells us how many protons are in the nucleus.
For example:
-
If an atom has 1 proton, it’s always hydrogen. No other element has 1 proton.
-
If an atom has 8 protons, it’s oxygen — always. Oxygen will never have 7 or 9 protons.
-
Fluorine has 9 protons → atomic number = 9
So:
Atomic number = number of protons = the identity of the element
Even if the atom gains or loses electrons, or becomes part of a compound — its atomic number stays the same, because the protons inside the nucleus don’t change.
That’s why the atomic number is so important in chemistry — it helps us identify atoms just like a name or fingerprint.
Tip for Remembering Atomic Number
The atomic number of an element is also its position in the Periodic Table.
That means:
-
The 1st element in the Periodic Table is Hydrogen, and its atomic number is 1
-
The 8th element is Oxygen, and its atomic number is 8
-
The 11th element is Sodium, and its atomic number is 11
Atomic number = the element’s order in the Periodic Table
What Is Mass Number? (Z)
The mass number tells us how heavy an atom is.
In real life, we measure weight using units like grams, kilograms, or milligrams. But as we already know, atoms are extremely small — so small that our normal weighing scales can’t measure them at all.
That’s why scientists use a different method to talk about the “mass” of atoms.
Instead of grams, we just count the number of particles inside the atom. And the ones that matter are:
-
Protons
-
Neutrons
These two particles are found in the nucleus, and they have mass.
So the formula is:
Mass number = number of protons + number of neutrons
But wait — what about electrons? Aren’t they part of the atom too?
Yes, they are! But electrons are so small and so light that their mass is almost nothing compared to protons and neutrons.
To help you understand this, imagine you place a sensitive digital scale in open air. Even though air is touching it, the scale doesn’t show any weight — because the air is too light to register.
That’s how small an electron’s mass is — we don’t include it when calculating the atom’s mass.
Examples: How to Calculate Mass Number
| Example 1: Helium (He) | Example 2: Carbon (C) |
|---|---|
| Protons: 2 Neutrons: 2 Electrons: 2 (not counted) Mass number = 2 + 2 = 4 |
Protons: 6 Neutrons: 6 Electrons: 6 (not counted) Mass number = 6 + 6 = 12 |
| Example 3: Oxygen (O) | Example 4: Sodium (Na) |
|---|---|
| Protons: 8 Neutrons: 8 Electrons: 8 (not counted) Mass number = 8 + 8 = 16 |
Protons: 11 Neutrons: 12 Electrons: 11 (not counted) Mass number = 11 + 12 = 23 |
Isotopes
Are Neutrons Unique for Each Atom?
Earlier, we learned that the atomic number is unique for every element. It tells us how many protons an atom has — and that number never changes.
But what about neutrons?
Do all atoms of the same element have the same number of neutrons?
The answer is: Not always.
Atoms of the same element can have different numbers of neutrons, even though they have the same number of protons.
This means that neutrons are not a fixed property of an element.
So while protons determine which element an atom is, the number of neutrons can vary — without changing the element itself.
What Are Isotopes?
When atoms of the same element have different numbers of neutrons, they are called isotopes.
-
All isotopes of an element have the same number of protons
-
But they have different atomic masses, because of the varying number of neutrons
This difference in mass doesn’t affect their chemical behavior isotopes still react the same way but it can affect their physical properties (like stability or radioactivity).
Example: Isotopes of Hydrogen
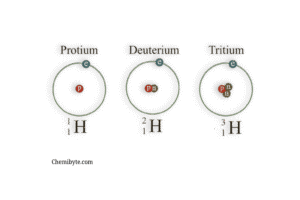
Let’s take hydrogen as an example.
Hydrogen is the first element in the Periodic Table and always has 1 proton. But it actually has three different isotopes — and they all are still hydrogen!
So why are there different types?
Because each one has a different number of neutrons in the nucleus.
| Isotope Name | Protons | Neutrons | Mass Number | Symbol |
|---|---|---|---|---|
| Protium | 1 | 0 | 1 | ¹H or H-1 |
| Deuterium | 1 | 1 | 2 | ²H or H-2 |
| Tritium | 1 | 2 | 3 | ³H or H-3 |
-
Protium is the most common form — it’s what you find in normal water.
-
Deuterium is heavier and is used in scientific research and nuclear reactors.
-
Tritium is radioactive and used in glow-in-the-dark devices and nuclear fusion experiments.
Even though they have different neutrons and mass numbers, all three are still hydrogen — because they all have 1 proton, and that’s what makes them hydrogen.
That’s the key idea of isotopes:
Same element, different versions
Do Isotopes Behave Differently?
Now you might be wondering:
If isotopes have different numbers of neutrons, does that change how they react in chemistry?
Here’s the simple answer:
Chemical properties stay the same
Because chemical reactions are all about electrons — and all isotopes of an element have the same number of protons and electrons they behave the same way in chemical reactions.
So:
-
Protium, Deuterium, and Tritium all react like hydrogen
-
Chlorine-35 and Chlorine-37 both form compounds like NaCl (salt)
Physical properties may change
But since isotopes have different mass numbers, their physical properties can be slightly different.
Examples:
-
Heavier isotopes may move slower in reactions
-
Their melting or boiling points might be slightly higher or lower
-
Tritium is radioactive — that’s a physical property that’s very different from Protium
Summary:
| Property | Do Isotopes Change It? | Why? |
|---|---|---|
| Chemical Properties | No | Electrons are the same |
| Physical Properties | Sometimes | Mass is different (neutron count) |
Practice Questions – Atoms, Subatomic Particles & Isotopes (GCSE/O-Level)
Question 1: Multiple Choice
Which of the following statements is true about protons?
A. They are found in shells
B. They have no charge
C. They are lighter than electrons
D. They are found in the nucleus and have a positive charge
✅ Answer: D
Explanation: Protons are located in the nucleus and carry a +1 charge. Electrons are found in shells and are much lighter.
Question 2: Fill in the Blanks
Complete the sentence:
The atomic number of an element tells us the number of _______ in one atom of that element.
✅ Answer: protons
Explanation: Atomic number = number of protons = identity of the element.
Question 3: Table Completion
Fill in the missing values in the table:
| Particle | Charge | Relative Mass | Location |
|---|---|---|---|
| Proton | ? | 1 | In the nucleus |
| Neutron | 0 | ? | In the nucleus |
| Electron | –1 | ~0 | ? |
✅ Answer:
| Particle | Charge | Relative Mass | Location |
|---|---|---|---|
| Proton | +1 | 1 | In the nucleus |
| Neutron | 0 | 1 | In the nucleus |
| Electron | –1 | ~0 | In shells (outside nucleus) |
Question 4: Short Answer
An atom has 6 protons, 6 neutrons, and 6 electrons.
a) What is the atomic number?
b) What is the mass number?
Answer:
a) 6 (atomic number = number of protons)
b) 12 (mass number = protons + neutrons = 6 + 6)
Question 5: Explain
Explain why atomic number can be used to identify an element, but the number of neutrons cannot.
Answer:
Atomic number is always equal to the number of protons, and this number is unique for each element. The number of neutrons can vary, so it cannot be used to identify the element. For example, chlorine atoms can have 18 or 20 neutrons but still be chlorine.
Question 6: Isotopes (Structured)
Hydrogen has three isotopes:
-
Protium: 1 proton, 0 neutrons
-
Deuterium: 1 proton, 1 neutron
-
Tritium: 1 proton, 2 neutrons
a) What is the atomic number of all three isotopes?
b) Which isotope has the highest mass number?
c) Why are all three considered hydrogen?
Answer:
a) 1 (they all have 1 proton)
b) Tritium (1 + 2 = 3)
c) Because they all have the same number of protons, which defines the element
Question 7: Calculation
An atom has:
-
Atomic number = 12
-
Mass number = 25
How many neutrons does it have?
Answer: 13 neutrons
Explanation: Neutrons = mass number – atomic number = 25 – 12
Challenging Practice Questions – Atoms, Structure & Isotopes
Question 8: Reasoning Question
Why do isotopes of an element have different mass numbers but the same chemical properties?
Explain your answer clearly.
Answer:
Isotopes have different mass numbers because they have different numbers of neutrons. However, they have the same number of protons and electrons, which means they behave the same way in chemical reactions. Chemical properties depend on electrons, not neutrons.
Question 9: Diagram Question
Below is an incomplete diagram of a lithium atom.
It has 3 protons, 3 neutrons, and 3 electrons.
a) Label the parts of the atom: nucleus, electrons, proton, neutron
b) Write the atomic number and mass number for lithium
c) Describe how the electrons are arranged in shells
Answer:
a) Label nucleus (center), show 3 protons (+), 3 neutrons (no charge), and 2 electrons in the first shell, 1 in the second
b) Atomic number = 3, Mass number = 6
c) Electrons are arranged as 2 in the first shell, 1 in the second → 2,1
Question 10: Explain with Comparison
Hydrogen-1 and Tritium are both forms of hydrogen. One is radioactive, and one is not. Explain why.
Answer:
Tritium (³H) has 2 neutrons, which makes its nucleus unstable — so it becomes radioactive. Hydrogen-1 (¹H) has no neutrons and is stable. The extra neutrons in Tritium cause it to decay over time, which is why it’s used in nuclear science and glow devices.
Question 11: Element Comparison
Two atoms are described below:
-
Atom A: 6 protons, 6 neutrons, 6 electrons
-
Atom B: 6 protons, 8 neutrons, 6 electrons
a) Are these atoms the same element?
b) Are they isotopes?
c) Which atom is heavier?
Answer:
a) Yes, they both have 6 protons → so both are carbon
b) Yes, because they have the same number of protons but different neutrons → they are isotopes of carbon
c) Atom B is heavier (6 + 8 = 14), while Atom A = 12
Question 12: Data-based Thinking
You are given 3 unknown atoms:
-
Atom X: 17 protons, 18 neutrons
-
Atom Y: 17 protons, 20 neutrons
-
Atom Z: 18 protons, 20 neutrons
a) Which atoms are isotopes?
b) Which one is a different element?
Answer:
a) Atom X and Atom Y are isotopes — both have 17 protons = chlorine
b) Atom Z has 18 protons = a different element (argon)
Key Takeaways
-
Atoms are the basic building blocks of matter.
-
Each atom has protons, neutrons, and electrons — called subatomic particles.
-
Protons decide the identity of an atom and are always equal to the atomic number.
-
Neutrons can vary — and when they do, the atom becomes an isotope.
-
Electrons orbit the nucleus in energy levels and are responsible for chemical reactions.
-
Mass number = protons + neutrons
-
Isotopes have the same chemical properties, but may have slightly different physical properties.
Glossary
-
Atom – The smallest unit of matter that makes up all substances
-
Proton – Positively charged particle in the nucleus
-
Neutron – Neutral particle in the nucleus
-
Electron – Negatively charged particle that orbits the nucleus
-
Atomic Number – The number of protons in an atom
-
Mass Number – The total number of protons and neutrons
-
Isotope – An atom of the same element with a different number of neutrons
Want more clear chemistry explanations? New lessons will be published soon—start at the Chemibyte home page: Chemibyte.com
Read more – Periodic Table of Elements
