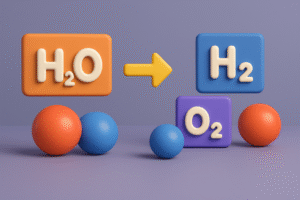Introduction – Why Do We Need Atomic Models?
Have you ever wondered what everything is really made of? The screen you’re reading this on, the air around you, even your own body – it’s all made of atoms.
But atoms are far too small to see, even with powerful microscopes. So, how do we understand what they’re like?
Over the last 200 years, scientists have created different atomic models to help explain what atoms are, what they’re made of, and how they behave. These models weren’t just random guesses – each one was based on experiments and observations that pushed our understanding forward.
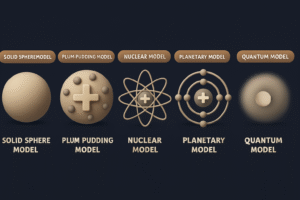
From Dalton’s solid spheres to Thomson’s plum pudding, Rutherford’s nucleus, and Bohr’s orbits, each model solved a problem the last one couldn’t explain.
In this post, we’ll take a simple, step-by-step journey through the five major atomic models and see how each one brought us closer to the atom’s true structure.
Let’s begin with the very first model Dalton’s.
Dalton’s Atomic Model (1803)
What Led to Dalton’s Idea
In the early 1800s, chemists began noticing something curious during their experiments. Whenever elements reacted to form compounds, they didn’t just mix randomly, they always combined in simple, fixed ratios.
Take water, for example. No matter where it comes from – a river, the ocean, or even created in a lab – it always breaks down into the same two elements: hydrogen and oxygen. And here’s the surprising part:
$$
\ce{2H2O(aq) -> H2(g) + O2(g)}
$$
- You always get twice as much hydrogen gas as oxygen gas when you split water using electricity.
This told scientists that water is made from 2 parts hydrogen to 1 part oxygen – no more, no less.
They saw the same thing with carbon dioxide:
$$
\ce{2CO2(g) -> 2C(s) + 2O2(g)}
$$
-
Every sample of carbon dioxide always had the same ratio – 1 part carbon to 2 parts oxygen.
No matter how many times they repeated the experiment, the numbers didn’t change.
That raised a much bigger question:
Why?
Why did elements always combine in such exact, predictable amounts?
This wasn’t just a detail — it felt like a clue.
That’s when a British scientist named John Dalton had an idea that pulled everything together.
What if, he thought, matter isn’t continuous or endlessly divisible…
What if everything is made of tiny, indivisible units — solid, invisible particles — that combine in set ways?
He called them atoms.
In 1803, Dalton introduced this bold new theory. It wasn’t just a guess — it explained why elements combine the way they do, and why those ratios never change. It gave structure to the chaos. It made chemistry make sense.
And just like that, a new way of understanding matter was born.
How Did Dalton Explain the Atom?
In 1803, John Dalton introduced a completely new idea about what matter is made of. Based on all the experiments and patterns scientists had observed, he came up with a simple but powerful theory:
Everything around us is made of tiny, invisible particles called atoms.
But Dalton didn’t just stop at that. He went further and explained how atoms behave and why they matter.
Here’s what he believed:
1. Atoms are tiny, solid particles
Dalton imagined atoms as small, solid balls, kind of like marbles. You can’t see them, and you can’t break them down any further. They’re the smallest possible pieces of matter.
2. Each element has its own type of atom
All atoms of a given element are identical to each other. For example, every oxygen atom is exactly like every other oxygen atom. But oxygen atoms are completely different from hydrogen atoms or carbon atoms.
3. Atoms combine in simple, whole-number ratios
When atoms join together to form compounds, they always do so in fixed ratios.
Like in water (H₂O) – two hydrogen atoms for every one oxygen atom. You never get half an atom. It’s always clean, simple numbers.
4. Atoms are not created or destroyed in reactions
In a chemical reaction, atoms don’t disappear or get created from nothing.
They just get rearranged. The number of atoms before the reaction is the same as after – they just form new combinations.
In Short:
Dalton’s atomic theory helped scientists understand why elements combine the way they do, and it laid the foundation for everything that came after – from the periodic table to modern chemistry.
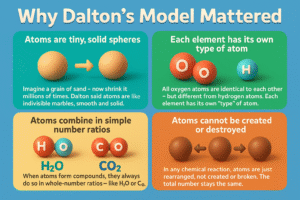
Why Dalton’s Model Mattered
Dalton’s ideas helped explain:
- Why chemical formulas have fixed numbers (like H₂O, CO₂)
- How to measure atomic weights of elements
- Why reactions follow conservation of mass
It was the first time anyone gave a scientific explanation for how matter behaves in chemical reactions.
What Dalton’s Model Couldn’t Explain
Like all first attempts, Dalton’s model had some gaps:
- He thought atoms were indivisible, but we now know they have protons, neutrons, and electrons inside.
- He didn’t know about isotopes atoms of the same element with different masses.
Still, Dalton laid the foundation of atomic theory and his model sparked more discoveries to come.
Thomson’s Plum Pudding Model (1897)
What Changed – A Surprising Discovery Inside the Atom
By the late 1800s, Dalton’s idea of atoms as solid balls was still popular. But in 1897, a British scientist named J. J. Thomson made a discovery that changed everything.
He was experimenting with cathode rays glowing beams that travelled through a sealed glass tube when electricity passed through. But something strange happened:
The rays bent toward a positively charged plate, which meant they had to be negatively charged.
That’s how Thomson discovered the electron – a tiny, negative particle inside the atom.
This was a big moment: for the first time, scientists realized that atoms weren’t solid all the way through. They had smaller parts inside them.
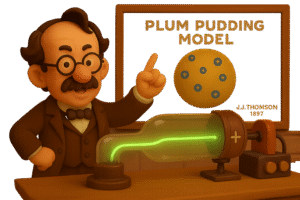
So he proposed a new model of the atom, which is known as the plum pudding model.
What Did the Plum Pudding Model Say?

After discovering the electron, Thomson needed a way to explain how these negative particles fit inside the atom.
So he came up with a new model: the plum pudding model.
-
He imagined the atom as a soft, positively charged sphere, like a pudding or cake.
-
The electrons were spread throughout it – like plums or raisins scattered in a dessert.
This was the first time anyone had suggested that atoms weren’t just solid lumps -but had positive and negative parts inside.
What This Model Got Right
-
It correctly introduced the idea of electrons – and that atoms are made of smaller particles.
-
It moved away from Dalton’s “solid sphere” idea and opened the door to exploring atomic structure.
What It Got Wrong
-
There was no nucleus – just a soft positive blob.
-
It couldn’t explain how electrons stayed in place, or how atoms held together.
-
It didn’t match what later experiments (like Rutherford’s) would show.
Why It Still Mattered
Even though the plum pudding model was eventually proven wrong, it was an important step forward. It showed that:
-
Atoms aren’t indivisible.
-
Electric charge plays a major role inside atoms.
Thomson’s model helped scientists ask new questions – including one that would lead to the next big breakthrough:
If electrons are scattered inside the atom… what’s at the center?
Let’s find out what happened next – in Rutherford’s gold foil experiment.
Rutherford’s Nuclear Model (1911)
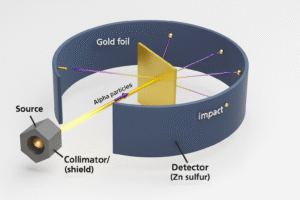
The Experiment That Changed Everything
In 1911, a scientist named Ernest Rutherford set out to test Thomson’s plum pudding model. If atoms were just soft balls with electrons mixed inside, particles should pass through them easily.
To find out, Rutherford and his team (including Geiger and Marsden) performed a now-famous experiment: the gold foil experiment.
Here’s what they expected:
The alpha particles should just pass straight through – like bullets through tissue paper because the existing atomic model (the Plum Pudding Model) said atoms were soft, spread-out blobs of positive charge.
But that’s not what happened.
What They Saw:
-
Most of the alpha particles passed straight through the foil – no problem.
-
Some particles got slightly deflected – like they hit something and bounced off at an angle.
-
A few bounced almost straight back, like they hit a wall!
So Why Did That Happen?
Here’s the logic Rutherford used to explain it:
1. Most went straight through –Because atoms are mostly empty space.
That’s why nothing stopped or redirected them.
2. Some particles deflected –Because they came close to something very dense and positively charged.
Since both the alpha particle and the center of the atom were positive, they repelled each other – like magnets pushing away.
3. A few bounced back – That meant they hit something really small but extremely dense something with enough mass and positive charge to knock them back.
That “something” turned out to be the nucleus.
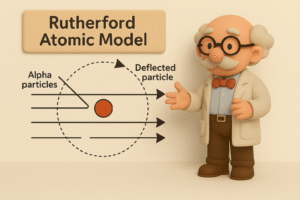
What Rutherford Discovered:
At the center of each atom is a tiny, dense, positively charged nucleus, and electrons orbit around it. The rest of the atom? It’s mostly empty space.
In Simple Words:
-
Most alpha particles went through because there was nothing in their way.
-
Some were pushed off course because they got close to the nucleus.
-
A few bounced back because they hit the nucleus head-on — which is tiny but powerful.
What Rutherford’s Model Got Right
-
Introduced the nucleus – a small, dense center inside the atom
-
Explained why most particles passed through, but some bounced back
-
Proved that atoms are mostly empty space
What It Couldn’t Explain
-
If electrons were circling the nucleus, why didn’t they spiral in and collapse?
-
It didn’t explain how atoms give off light or energy during reactions
-
It couldn’t describe why electrons stay in place
Still, Rutherford’s model brought us much closer to the real structure of atoms – and laid the foundation for the next big idea: Bohr’s atomic orbits.
Bohr’s Model (1913)
The Problem with Rutherford’s Atom
Rutherford’s model introduced the idea of a nucleus, which was a big breakthrough. But there was still one major problem:
If electrons were constantly moving around the nucleus – like planets around the sun – they should lose energy and eventually spiral inward and crash into the nucleus.
But atoms in real life are stable. They don’t collapse. So… why not?
This mystery was solved by a young Danish physicist named Niels Bohr.
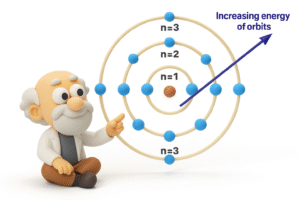
What Bohr Suggested
In 1913, Bohr proposed a new idea:
What if electrons don’t move just anywhere – but only in fixed paths or energy levels?
Here’s what his model said:
-
Electrons move in circular orbits around the nucleus.
-
These orbits are called energy levels or shells.
-
Each level has a specific amount of energy.
-
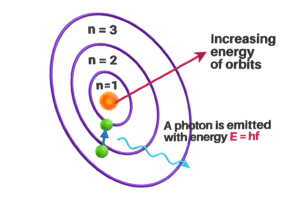
Electrons can jump from one level to another – but only by absorbing or releasing energy.
- When electron jump from a lower level to higher level – it absorb energy and when electron jump from a higher level to a lower level – it releases energy.
This model explained why atoms don’t collapse – because electrons are only allowed in certain stable orbits.
What Bohr’s Model Got Right
-
Explained why atoms are stable
-
Matched up with light spectra (like the colored lines seen when elements are heated)
-
Introduced the idea of quantized energy – energy that comes in fixed amounts
-
Still used in Schools (GCSE Chemistry) today to describe atomic structure
What Bohr’s Model Couldn’t Explain
-
It worked really well for hydrogen, but not for larger atoms
-
It didn’t include electron clouds or probability zones (we use those in modern quantum models)
-
It didn’t explain chemical bonding in detail
Why It Still Matters
Even though newer models came later, Bohr’s model is still:
-
The easiest to understand for beginners
-
A great way to explain electron shells, atomic numbers, and ion formation
-
The foundation for many chemistry basics, including the Periodic Table and chemical reactions
Bohr’s model helped bring everything together and made it possible to start explaining the behavior of atoms using both energy and structure.
Next, we’ll wrap up with one last piece of the puzzle: the discovery of the neutron.
Chadwick’s Discovery of the Neutron (1932)

The Missing Piece in the Atomic Puzzle
By the 1920s, Bohr’s model gave a clear picture of electrons moving in orbits, and Rutherford had shown that atoms have a positively charged nucleus made of protons.
But something still didn’t add up.
When scientists measured the mass of atoms, they noticed it was heavier than expected. For example:
-
Helium has 2 protons, so it should have a mass of 2, right?
-
But its mass was around 4.
Where was the extra mass coming from?
That’s when James Chadwick, a British physicist, began to investigate further.
What Chadwick Discovered
In 1932, Chadwick carried out experiments where he bombarded atoms with particles and measured what came out.
What he found was a new kind of particle:
-
It had mass, like a proton
-
But it had no charge
He named this neutral particle the neutron.
Neutrons lived inside the nucleus, alongside protons – and their presence explained the missing mass in atoms.
So the modern view of the nucleus became:
A dense center made of both protons and neutrons.
Why Neutrons Matter
-
They add mass to atoms
-
They help hold the nucleus together (protons would repel without them)
-
They explain the existence of isotopes – atoms of the same element with different numbers of neutrons
For example:
-
Carbon-12 has 6 protons and 6 neutrons
-
Carbon-14 has 6 protons and 8 neutrons
Same element, different mass – thanks to neutrons.
The Atom – As We Know It Today
With Chadwick’s discovery, the basic structure of the atom became:
-
A nucleus made of protons and neutrons
-
Electrons in energy levels (or orbitals) around the nucleus
Later on, more complex quantum models would replace fixed orbits with electron clouds, but for GCSE and early chemistry, this model remains the core idea.
What Is the Current Atomic Model – And Who Discovered It?
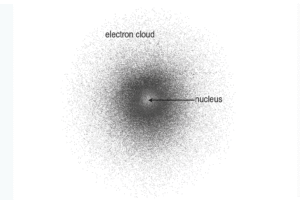
Today, scientists use the Quantum Mechanical Model of the Atom to explain how atoms work.
This model doesn’t show electrons moving in neat orbits (like Bohr suggested). Instead, it says:
-
Electrons exist in cloud-like regions around the nucleus
-
We can’t know their exact position and speed at the same time
-
These regions are called orbitals, and they have different shapes (not just circles)
-
Electrons behave like both particles and waves
Who Developed the Quantum Model?
The quantum model wasn’t invented by just one person – it came from the work of several scientists in the 1920s:
-
Erwin Schrödinger (Austria)
Developed the mathematical wave equation that predicts where electrons are likely to be
→ His model is often called the Electron Cloud Model. -
Werner Heisenberg (Germany)
Proposed the Uncertainty Principle – we can’t know both the position and speed of an electron exactly -
Max Planck and Niels Bohr also contributed by developing early ideas about quantum energy levels
Together, their work created the modern understanding of atoms used in advanced chemistry and physics today.
Why These Atomic Models Still Matter
Even though science has moved beyond these early models, they still help us:
-
Understand the structure of matter at a basic level
-
Explain chemical formulas, bonding, and reactions
-
See how science evolves: each model fixed the problems of the one before it
In most school-level chemistry (like GCSE or O-Level), we still use Bohr’s model to explain:
-
Atomic number and mass number
-
Electron shells and configurations
-
Ion formation and periodic table trends
Understanding these models isn’t just about memorizing history – it’s about seeing how scientists build knowledge over time.
Practice Questions – Atomic Models
Try these questions to check your understanding:
-
Who first suggested that atoms are tiny, indivisible spheres?
-
What did J. J. Thomson discover about the atom?
-
How did Rutherford’s experiment change the plum pudding model?
-
What key idea did Bohr add to atomic theory?
-
Why was Chadwick’s discovery important?
-
Which model first introduced the nucleus?
-
What do we mean by “energy levels” in Bohr’s model?
-
What are isotopes and which particle explains them?
Key Takeaways – Evolution of Atomic Models
-
Dalton introduced atoms as indivisible solid spheres.
-
Thomson discovered the electron and proposed the plum pudding model.
-
Rutherford showed that atoms have a tiny, dense nucleus.
-
Bohr introduced energy levels, explaining atomic stability.
-
Chadwick completed the model by discovering the neutron.
Each model built on the last – helping us understand what atoms are, how they behave, and how matter works.
Glossary
-
Atom – The smallest unit of matter; made of protons, neutrons, and electrons.
-
Electron -A negatively charged particle that orbits the nucleus.
-
Proton – A positively charged particle found in the nucleus.
-
Neutron – A neutral (no charge) particle in the nucleus; adds mass and stability.
-
Nucleus – The central part of an atom containing protons and neutrons.
-
Energy levels – Fixed paths or shells where electrons are likely to be found.
-
Isotopes – Atoms of the same element with different numbers of neutrons.
Still have a question?
Drop it below
Written by RK Aera
I create calm, clear science blogs to help students and curious learners around the world.
Explore more posts at chemibyte.com