Electrons, Energy Levels & Orbitals – GCSE Guide
Two Ways to Picture Electrons (Bohr vs the Quantum Model)
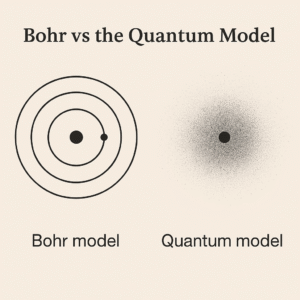
If electrons feel mysterious, you’re not alone. Textbooks throw around words like shells, orbitals, and spin as if everyone was born knowing them. This first part gives you a friendly mental picture that actually sticks—so the rest of the topic feels doable.
The Bohr Model: the tidy, first‑step picture
Imagine the atom like a tiny solar system. In Niels Bohr’s early model, electrons sit in fixed energy levels (called shells) around the nucleus – like lanes on a running track. The idea is simple and useful:
-
Shells are labelled n = 1, 2, 3, 4…
-
Higher shells are further from the nucleus and have more energy.
-
For the first 20 elements, many courses summarise electron counts with the neat 2,8,8 pattern across shells.
It’s a great starting point because it explains a lot of early periodic trends without too much maths.
Exam tip: Some GCSE specs mainly test this shell model for the first 20 elements. Others include orbital notation as extension/triple content. It’s worth knowing both, but check your spec so you revise the right depth.
The Quantum Model: the accurate, modern picture
Here’s where reality gets less “solar system” and more “probability.” Electrons don’t whizz in perfect circles. Instead, they occupy orbitals—regions of space where an electron is most likely to be found.
-
Orbitals live inside shells and come in types like s, p, d, f (at GCSE you’ll mostly meet s and p).
-
Each orbital can hold up to 2 electrons, and those two must have opposite spins.
-
“Spin” isn’t literal clockwise vs anticlockwise – it’s a quantum property. We just draw it as ↑ and ↓ to show they’re different.
Think of orbitals as “electron clouds”: not hard edges, but regions where the electron spends most of its time.
How the two pictures work together
You don’t have to choose one model and dump the other. For GCSE chemistry, it’s smartest to use both:
-
Shells (Bohr) = the big picture of energy levels.
-
Orbitals (quantum) = the detail inside each shell (how many electrons fit, how they pair, and the shapes that influence chemistry).
This combined view is powerful. It explains why some shells split into subshells, why we write configurations like 1s² 2s² 2p⁶, and even why certain elements behave the way they do.
A simple analogy that actually helps
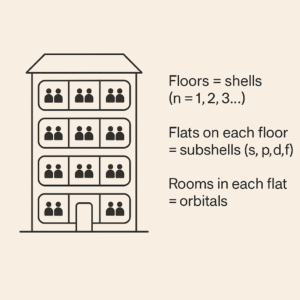
Picture an apartment building:
-
Floors = shells (n = 1, 2, 3…)
-
Flats on each floor = subshells (s, p, d, f)
-
Rooms in each flat = orbitals
-
People = electrons
Every room (orbital) can hold two people – but they must be “opposites” (↑ and ↓). Higher floors are further up (more energy). Some floors have more flats than others. Suddenly, the layout of electrons starts to make everyday sense.
Pitfalls to dodge from day one
-
Electrons are not tiny planets on circular tracks.
-
Orbital diagrams show probability regions, not hard‑walled rooms.
-
“Spin” is a label we use to keep track of two allowed states – not a literal twirl.
Electrons are tiny, but understanding where they sit in energy levels and atomic orbitals is a big deal in GCSE Chemistry. These ideas appear again and again in exams, from shells and sub-shells to electron configurations. In this guide, we keep it simple with clear rules, clean diagrams, and step-by-step examples, so you can remember it easily when revising.
Energy levels & Orbitals
Energy Levels (shells)
Welcome to the “floors of the building.” Shells are the broad energy levels that organise where electrons can go. Get this picture clear and everything else – subshells, orbitals, configurations- slots into place.
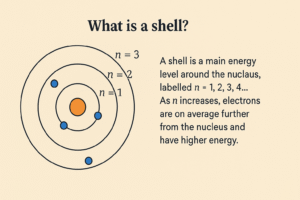
What is a shell?
-
A shell is a main energy level around the nucleus, labelled n = 1, 2, 3, 4…
-
As n increases, electrons are on average further from the nucleus and have higher energy.
-
Think floors in a tower: floor 1 is closest to the lobby (nucleus), higher floors are further away and “cost” more energy.
How many electrons can a shell hold?
-
Maximum capacity (in theory) follows 2n²:
-
n = 1: 2 × 1² = 2
-
n = 2: 2 × 2² = 8
-
n = 3: 2 × 3² = 18
-
n = 4: 2 × 4² = 32
-
-
GCSE nuance: For the first 20 elements, you’ll see the pattern 2,8,8,2.
That’s because, in real atoms, the 4s subshell begins to fill before the 3d subshell—so shell 3 looks “full at 8” for a while. (We’ll unpack this in the filling‑order part.)
Exam tip: In simple shell diagrams for Z ≤ 20, count electrons across shells as 2, 8, 8, (up to) 2. Save 3d for later.
Periods, groups, and shells
-
Period number (row of the periodic table) = number of occupied shells.
-
Example: Magnesium (Mg), Z=12 → 2,8,2 → Period 3 (three occupied shells).
-
-
For main‑group (s‑ and p‑block) elements, the group number often matches the number of outer‑shell (valence) electrons (with the usual caveats for the older vs newer group numbering).
First‑20 wall chart (shell model view)
Use this to sanity‑check your answers fast:
-
H -1
-
He – 2
-
Li – 2,1
-
Be – 2,2
-
B – 2,3
-
C – 2,4
-
N – 2,5
-
O – 2,6
-
F -2,7
-
Ne – 2,8
-
Na – 2,8,1
-
Mg – 2,8,2
-
Al – 2,8,3
-
Si – 2,8,4
-
P – 2,8,5
-
S – 2,8,6
-
Cl – 2,8,7
-
Ar – 2,8,8
-
K – 2,8,8,1
-
Ca – 2,8,8,2
(We’ll translate these into orbital notation—1s² 2s² 2p⁶ etc.—in the next parts.)
Common traps (and how to dodge them)
-
Trap: “Shell 3 always holds 18 in GCSE.”
Fix: It can hold 18 in theory (2n²), but up to argon you usually see 8 because 4s fills before 3d. -
Trap: “K and Ca use 3d before 4s.”
Fix: 4s fills first, so K = 2,8,8,1 and Ca = 2,8,8,2. -
Trap: Mixing shell counts with orbital counts.
Fix: Shells are the big levels; orbitals are the rooms inside (we’ll get to those next).
30‑second self‑check
-
Which shell is the valence shell in Sulfur (S, Z=16)?
-
How many occupied shells in Aluminium (Al, Z=13)?
-
Give the shell model for Potassium (K, Z=19).
Answers:
-
S (2,8,6) → n = 3 is the valence shell.
-
Al (2,8,3) → 3 occupied shells.
-
K → 2,8,8,1.
Subshells: s, p, d, f (what lives inside each shell)
So you’ve got the floors of the building (shells). Now let’s step into the flats on each floor – the subshells. This is where the layout starts to explain why certain elements behave alike and how we pack electrons in an order you can actually memorise.
What is a subshell?
A subshell is a subdivision of a shell with a slightly different energy. Subshells are labelled s, p, d, f and they appear in a predictable way:
-
n = 1 –>1s
-
n = 2 –> 2s, 2p
-
n = 3 –> 3s, 3p, 3d
-
n = 4 –> 4s, 4p, 4d, 4f
GCSE scope: You rarely need beyond n = 4. For the first 20 elements, you’ll mostly use s and p (with 4s arriving before 3d-we’ll lean on that order soon).
How many electrons can each subshell hold?
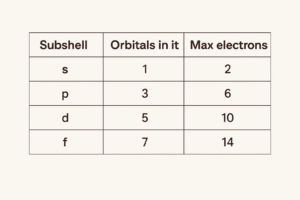
Each subshell contains a certain number of orbitals (rooms). Every orbital holds up to 2 electrons. Here’s the whole set at a glance:
Memory hook (works every time):
Orbitals per subshell go 1, 3, 5, 7 (odd numbers). Double it to get electrons: 2, 6, 10, 14.
Why do shells “split” into subshells?
Short answer for GCSE: energy fine‑structure. Electrons feel different amounts of attraction to the nucleus and repulsion from other electrons depending on the shape of the region they occupy. That’s why the shell divides into s, p, d, f with slightly different energies.
Shapes you’ll actually use
You’ll meet the shapes properly in the next part (orbitals), but two headlines help now:
-
s subshell → 1 spherical orbital
-
p subshell → 3 dumbbell‑shaped orbitals (pₓ, pᵧ, p_z)
(d and f are more complex—just know they exist and explain the transition metals and lanthanides/actinides.)
Subshells and the periodic table (the “blocks”)
A neat bonus: the periodic table is built around which subshell is filling:
-
s‑block: Groups 1–2 (and helium by electrons)
-
p‑block: Groups 13–18
-
d‑block: Transition metals (where 3d, 4d, 5d fill)
-
f‑block: The bottom rows (where 4f, 5f fill)
This is why elements in the same block often share patterns in their electronic configurations and chemical behaviour.
Mini‑examples (connect shells → subshells quickly)
-
Neon (Ne, Z=10): 1s² 2s² 2p⁶ (shell 2 contains s and p, total 8)
-
Magnesium (Mg, Z=12): 1s² 2s² 2p⁶ 3s²
-
Phosphorus (P, Z=15): 1s² 2s² 2p⁶ 3s² 3p³
Exam tip: Write subshells in the filling order you’ll memorise soon:
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p
For the first 20 elements, you won’t actually use 3d yet.
Common pitfalls (and quick fixes)
-
Pitfall: “p has two orbitals.” → Fix: p has three (px, py, pz).
-
Pitfall: “3d fills before 4s.” → Fix: For K and Ca, 4s fills first.
-
Pitfall: “Subshell capacity is random.” → Fix: Use the 1,3,5,7 → 2,6,10,14 rule.
60‑second practice
-
How many electrons can the 3p subshell hold?
-
Which subshells exist in the n = 3 shell?
-
True/False: The 2p subshell has two orbitals.
Answers:
-
6 (3 orbitals × 2 electrons)
-
3s, 3p, 3d
-
False—it has three (px, py, pz).
Atomic Orbitals: what they are and how to picture them
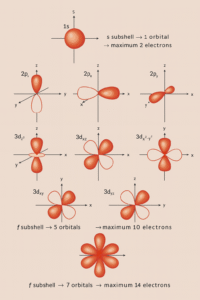
If shells are the floors and subshells are the flats, orbitals are the rooms where electrons actually “live.” Getting a feel for orbitals makes the arrow‑in‑boxes diagrams (and the filling order) make sense instead of feeling like magic.
So… what is an orbital?
An orbital is a region of space where there’s a high probability of finding an electron. It’s not a hard‑edged box or a tiny circular track; think of it like a cloud that shows where the electron spends most of its time.
-
Orbitals belong to subshells (s, p, d, f).
-
One orbital holds up to 2 electrons.
-
Those two electrons must have opposite spins (we draw them as ↑ and ↓).
This is the Pauli Exclusion Principle – no two electrons in the same orbital can be identical in all their quantum “labels,” so the spins must differ.
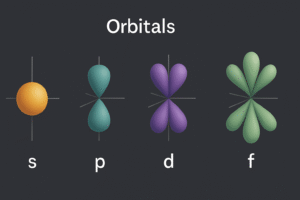
The shapes you need to know (GCSE‑core)
s orbital → spherical
-
Looks like a ball centered on the nucleus.
-
Every s subshell (1s, 2s, 3s…) has one s orbital.
p orbitals → dumbbells
-
Three of them at each p subshell: pₓ, pᵧ, p_z.
-
They point along the x, y, and z axes at right angles to each other.
-
Each p orbital is a two‑lobed “dumbbell” with a node (a thin region in the middle) at the nucleus.
d and f orbitals → complex (extension awareness)
-
Most d orbitals look like four‑leaf clovers; one (d_z²) is “dumbbell + ring.”
-
f orbitals are even more intricate.
-
For GCSE, you mainly need to know they exist—they explain the transition metals and the f‑block elements.
Try‑at‑home sketch: Draw a small circle for an s orbital. For p orbitals, draw three dumbbells: one horizontal (pₓ), one vertical (pᵧ), one coming “out of the page” (p_z). Label them. That picture will save you marks later.
Orbitals of equal energy (a key idea for arrows‑in‑boxes)
Within the same subshell, orbitals are equal in energy (we say degenerate).
-
-
Example: the three 2p orbitals (2pₓ, 2pᵧ, 2p_z) are all the same energy.
-
This sets up Hund’s rule:
Hund’s rule (student version): In a set of equal‑energy orbitals, put one electron in each orbital first with parallel spins (↑ ↑ ↑), and only then start pairing (↑↓).
You’ll use this rule every time you draw p or d subshells with arrows.
Orbitals → boxes and arrows (a gentle preview)
-
-
We often draw one box per orbital.
-
We place arrows for electrons: ↑ and ↓ for opposite spins.
-
For a p subshell, you always draw three boxes side by side.
-
First pass: fill ↑ ↑ ↑ across the three boxes (Hund’s rule).
-
Second pass: go back and pair ↓ where needed.
-
You’ll get a full workout with this in the “Electrons‑in‑Boxes” part, but this preview ties the shapes to what you draw.
Why do shapes matter at all?
Shapes control overlap and directionality when atoms bond, which affects real‑world properties (like molecular shapes and polarity). At GCSE, you won’t go deep into bonding overlap maths, but knowing s is spherical and p is directional helps later topics click.
Common pitfalls (fix them now)
-
“Spin = clockwise vs anticlockwise.”
Correct – Not literal spinning. It’s a quantum property. Just use ↑ and ↓. -
“2p has two orbitals.”
Correct – It has three: pₓ, pᵧ, p_z. -
“You can cram 3 electrons into one orbital if you try.”
Correct – Maximum two, and they must be opposite spins (Pauli).
45‑second practice
-
How many electrons fit in one orbital? Explain the spin condition briefly.
-
Name the three 2p orbitals. Are they the same energy?
-
Sketch arrows for 2p³ following Hund’s rule.
Answers (tap to check yourself):
-
Two, with opposite spins (↑↓), due to the Pauli Exclusion Principle.
-
2pₓ, 2pᵧ, 2p_z—yes, they’re degenerate (equal energy).
-
[↑] [↑] [↑] across three boxes (one in each, parallel spins).
What to remember from Part 4
-
Orbitals are probability regions, not tiny orbits.
-
Max 2 electrons per orbital, with opposite spins (Pauli).
-
In equal‑energy sets (like p), fill singly with parallel spins first (Hund).
How Many Electrons Fit in one Orbital? (the capacities you must memorise)
This is the part that makes electron configurations feel predictable instead of guesswork. If you remember the capacities below, you can sanity‑check any configuration in seconds.
The golden table
| Subshell | Orbitals in it | Max electrons (2 per orbital) |
|---|---|---|
| s | 1 | 2 |
| p | 3 | 6 |
| d | 5 | 10 |
| f | 7 | 14 |
Memory hook: orbitals go 1, 3, 5, 7 (odd numbers). Double them to get electrons: 2, 6, 10, 14.
Say it out loud once or twice—“s2, p6, d10, f14.”
Quick connections that help in exams
-
Neon (Ne, Z=10): shell 2 has 2s (2) + 2p (6) → total 8 in that shell → 1s² 2s² 2p⁶.
-
Argon (Ar, Z=18): add 3s (2) + 3p (6) → 1s² 2s² 2p⁶ 3s² 3p⁶.
-
Magnesium (Mg, Z=12): 3s holds 2, so … 3s² (no 3p yet).
Sanity check trick: Whenever you finish a p subshell, look for p⁶. A full s is s². If you ever write p⁷ or s³, something’s off.
Why the periodic table looks the way it does (the “blocks”)
-
s‑block (Groups 1–2) is 2 columns wide → s holds 2 electrons.
-
p‑block (Groups 13–18) is 6 columns wide → p holds 6 electrons.
-
d‑block (transition metals) is 10 columns wide → d holds 10 electrons.
-
f‑block (lanthanides/actinides) is 14 columns wide → f holds 14 electrons.
Once you see that, the table stops looking random.
The shell‑3 “8 vs 18” nuance (know this phrasing)
-
In theory, shell 3 can hold up to 18 electrons (because it has 3s, 3p, 3d).
-
For the first 20 elements, it appears to fill to 8 because the 4s subshell begins to fill before 3d.
-
That’s why K and Ca go into 4s next, and the 3d series starts with Sc.
Exam tip: For Z ≤ 20, the shell model you often use is 2, 8, 8, (up to) 2. In orbital notation, remember the filling order you’ll use shortly: … 3p → 4s → 3d.
Common pitfalls (and the fix)
-
“p has two orbitals.”
Correct – It has three (pₓ, pᵧ, p_z) → total 6 electrons. -
“d holds 8.”
Correct – d has 5 orbitals → 10 electrons. -
“You can stack 3 electrons in one orbital.”
Correct – Max 2 per orbital, and they must have opposite spins (↑↓).
60‑second practice
-
How many electrons can the 3d subshell hold?
-
How many orbitals are there in the 4f subshell?
-
If a student writes 2p⁷, what’s the error?
-
Which blocks of the periodic table correspond to 3d and 4f filling?
Answers:
-
10 (5 orbitals × 2)
-
7 orbitals
-
p can only hold up to 6 electrons; 2p⁷ is impossible.
-
3d → d‑block (transition metals), 4f → f‑block (lanthanides)
What to remember from Part 5
-
s2, p6, d10, f14—tattoo this on your brain.
-
Use s² and p⁶ as “full‑stop” checkpoints when writing configurations.
-
The periodic table’s block widths match subshell capacities – a brilliant shortcut when you’re under time pressure.
The Filling Order of electrons (Aufbau): the one line to memorise
Here’s the bit that turns configurations from guesswork into a recipe. Electrons go into the lowest‑energy orbitals first. For GCSE you only need a short stretch of the full sequence—memorise this exactly:
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p
That’s it. If you can say it out loud smoothly, you can build (and check) almost any configuration you’ll meet at this level.
Why that order?
-
Aufbau principle: fill lowest energy first.
-
Pauli: max 2 electrons per orbital, opposite spins (↑↓).
-
Hund: in equal‑energy sets (like the three p orbitals), fill singly with parallel spins before pairing (↑ ↑ ↑ then come back to pair).
You’ve already seen Pauli and Hund; here they simply tell you how to place arrows once you know which subshell fills next.
The quick visual (diagonal guide)
You don’t have to memorise this grid, but it helps you see why 4s sneaks in before 3d:
Draw diagonals from top‑right to bottom‑left and read along each arrow:
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s …
For GCSE, stop at 4p (and optionally 5s) unless your teacher gives you specific transition‑metal examples.
GCSE essentials you’ll actually use
-
Up to argon (Z=18) you’ll see: 1s² 2s² 2p⁶ 3s² 3p⁶.
-
Potassium (Z=19) and calcium (Z=20) go into 4s next:
-
K: … 4s¹
-
Ca: … 4s²
-
-
The 3d subshell starts at scandium (Z=21):
-
Sc: … 4s² 3d¹
-
(We’ll unpack the “why 4s before 3d” more in a later part; this is the usable order for now.)
Build‑it‑fast examples
Nitrogen (Z=7)
Order: 1s, 2s, 2p → fill 7 electrons → 1s² 2s² 2p³
(2p arrows: [↑] [↑] [↑])
Phosphorus (Z=15)
Order through 3p → 1s² 2s² 2p⁶ 3s² 3p³
(3p arrows: [↑] [↑] [↑])
Potassium (Z=19)
Go past 3p to 4s before 3d →
1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹
Calcium (Z=20)
Same, but 4s² →
1s² 2s² 2p⁶ 3s² 3p⁶ 4s²
Scandium (Z=21)
After filling 4s², next electron goes to 3d →
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹
Shorthand reminder:
P: [Ne] 3s² 3p³
K: [Ar] 4s¹
Ca: [Ar] 4s²
Sc: [Ar] 4s² 3d¹
Common pitfalls (fix them now)
-
Writing 3d before 4s for K/Ca → Remember the sequence: … 3p → 4s → 3d.
-
Overfilling a subshell (e.g., 2p⁷) → p tops out at p⁶.
-
Pairing too early in p → Use Hund:
[↑] [↑] [↑]before any[↓].
60‑second drill
-
Give the full configuration for Mg (Z=12) using the order above.
-
Which subshell comes after 4s in the GCSE list?
-
Where do the next two electrons go after [Ar] 4s²?
Check:
-
1s² 2s² 2p⁶ 3s²
-
3d (so the sequence is … 3p → 4s → 3d → 4p)
-
Into 3d (so Sc is … 4s² 3d¹, Ti is … 4s² 3d²)
What to remember from Part 6
-
Memorise the line: 1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p.
-
Apply Pauli (↑↓, max 2 per orbital) and Hund (fill singly with parallel spins).
-
For K and Ca, it’s 4s before 3d—then 3d starts at Sc.
Writing Electronic Configurations (full, shorthand, and fast checks)
This is the pay-off. With the filling order in your head, you can write configurations quickly and spot mistakes before they cost marks.
The recipe (every time)
-
Know the order: 1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p
-
Count up electrons to the element’s atomic number Z.
-
Fill each subshell to its max (s², p⁶, d¹⁰…) until you’ve placed Z electrons.
-
Stop—don’t overfill a subshell (no p⁷, no s³).
-
(Optional) Convert to shorthand using the nearest noble gas in square brackets.
Core examples (build them out loud)
Helium, He (Z = 2)
Order: 1s → stop.
Full: 1s²
Shorthand: He is itself a noble gas, so just 1s².
Beryllium, Be (Z = 4)
1s² 2s² → 1s² 2s²
Shorthand: [He] 2s²
Oxygen, O (Z = 8)
1s² 2s² 2p⁴ → 1s² 2s² 2p⁴
Shorthand: [He] 2s² 2p⁴
Magnesium, Mg (Z = 12)
1s² 2s² 2p⁶ 3s² → 1s² 2s² 2p⁶ 3s²
Shorthand: [Ne] 3s²
Phosphorus, P (Z = 15)
1s² 2s² 2p⁶ 3s² 3p³ → 1s² 2s² 2p⁶ 3s² 3p³
Shorthand: [Ne] 3s² 3p³
Potassium, K (Z = 19)
… 3p⁶ then 4s¹ → 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹
Shorthand: [Ar] 4s¹
Calcium, Ca (Z = 20)
… 4s² → 1s² 2s² 2p⁶ 3s² 3p⁶ 4s²
Shorthand: [Ar] 4s²
Scandium, Sc (Z = 21)
After 4s², next electron goes into 3d → … 4s² 3d¹
Shorthand: [Ar] 4s² 3d¹
Electrons-in-boxes: what your arrows should look like
Use one box per orbital; arrows show electrons and spin.
Oxygen (Z=8): 1s² 2s² 2p⁴
-
1s:
[↑↓] -
2s:
[↑↓] -
2p (three boxes): apply Hund
-
Fill singly first:
[↑] [↑] [↑] -
4th electron pairs one box:
-
Phosphorus (Z=15): 3p³
Hund’s rule reminder: in equal-energy orbitals (like the three p’s), fill one in each box first with parallel spins (↑ ↑ ↑), then pair.
Ions (quick but high-value)
-
Na → Na⁺: loses the outer 3s¹ → [Ne]
-
Mg → Mg²⁺: loses 3s² → [Ne]
-
Transition metals: remove from 4s before 3d
-
Fe (Z=26): [Ar] 4s² 3d⁶
-
Fe²⁺: [Ar] 3d⁶
-
Fe³⁺: [Ar] 3d⁵
-
-
Fast self-checks (catch mistakes early)
-
Finished an s? It should read s² (never s³).
-
Finished a p? It should read p⁶ (never p⁷).
-
For K and Ca, you must see 4s before any 3d.
-
Total electrons must equal Z (count them!).
90-second practice
-
Write full and shorthand configurations for Al (Z=13) and Cl (Z=17).
-
Draw electrons-in-boxes for N (Z=7) focusing on 2p.
-
State which subshell loses electrons first when Ti (Z=22) forms Ti²⁺.
Check yourself:
-
Al: 1s² 2s² 2p⁶ 3s² 3p¹ → [Ne] 3s² 3p¹
Cl: 1s² 2s² 2p⁶ 3s² 3p⁵ → [Ne] 3s² 3p⁵ -
N has 2p³ →
\[↑] \[↑] \[↑](one in each box, parallel) -
4s loses electrons before 3d → Ti²⁺ is [Ar] 3d²
What to remember from Part 7
-
Follow the order, cap subshells correctly, and count to Z.
-
Use shorthand to keep long configs neat.
-
For ions of transition metals, remove 4s before 3d.
Electrons-in-Boxes (clean diagrams examiners love)
This is where your configurations turn into quick, mark-winning sketches. The goal: draw tidy boxes and arrows that prove you understand Pauli + Hund and the filling order.
The ground rules (burn these in)
-
One box = one orbital.
-
Max 2 arrows per box, and they must point opposite ways (↑↓) → Pauli Exclusion Principle.
-
In sets of equal-energy orbitals (like p: three boxes; d: five boxes), fill one arrow per box first with the same direction (↑ ↑ ↑ …), then go back and pair → Hund’s rule.
Tip: Keep all your first arrows upwards (↑). When you come back to pair, add down (↓). Examiners like consistency.
How many boxes?
-
s: 1 box
-
p: 3 boxes (px, py, pz)
-
d: 5 boxes
-
f: 7 boxes (rare at GCSE)
Core p-subshell examples (the ones you’ll actually draw)
Nitrogen, N (Z=7) → … 2p³
Why: Three electrons, three boxes → one in each, parallel spins (Hund).
Oxygen, O (Z=8) → … 2p⁴
Why: First pass ↑ ↑ ↑, fourth electron pairs one box.
Fluorine, F (Z=9) → … 2p⁵
Neon, Ne (Z=10) → … 2p⁶
A couple of 3p examples (Period 3 practice)
Phosphorus, P (Z=15) → … 3p³
Chlorine, Cl (Z=17) → … 3p⁵
Peek at a d-subshell (useful for extension / transition-metal ions)
Scandium, Sc (Z=21) → … 4s² 3d¹
Iron, Fe (Z=26) → [Ar] 4s² 3d⁶
Fill 3d singly across five boxes (↑ ↑ ↑ ↑ ↑), then the 6th pairs the first box:
Ionisation tip: remove from 4s before 3d.
Fe²⁺: [Ar] 3d⁶ (the two 4s electrons are gone)
Fe³⁺: [Ar] 3d⁵ (one more removed from 3d)
Tidy-layout checklist (for full marks)
-
Draw all the boxes for that subshell (e.g., always three for p).
-
Keep arrow lengths and spacing neat; label the subshell (e.g., “2p”).
-
First fill ↑ across, then pair with ↓.
-
Don’t overfill (no p⁷, s³). Cap at s², p⁶, d¹⁰.
60-second drill
-
Draw 2p for B (Z=5), C (Z=6), N (Z=7).
-
Draw 3p for S (Z=16).
-
For Ti (Z=22) → [Ar] 4s² 3d², sketch 3d.
-
Which subshell loses electrons first when V → V²⁺?
Answers:
-
B (2p¹):
[↑ ] [ ] [ ]• C (2p²):[↑ ] [↑ ] [ ]• N (2p³):[↑ ] [↑ ] [↑ ] -
S (3p⁴):
[↑↓] [↑ ] [↑ ] -
Ti 3d²:
[↑ ] [↑ ] [ ] [ ] [ ] -
4s loses electrons before 3d.
What to remember from Part 8
-
Boxes = orbitals, 2 arrows max, opposite spins (Pauli).
-
In p or d sets, spread out first (Hund), then pair.
-
Keep diagrams labelled and symmetrical—it’s free clarity points.
Why 4s fills before 3d (and why 4s empties first)
This is the bit everyone asks about. You’ve memorised the order … 3p → 4s → 3d → 4p—but why does 4s sneak in before 3d? And why, when atoms turn into ions, do we remove 4s electrons first? Here’s the GCSE-safe story you can quote in an exam, plus a short extension if you’re curious.
The GCSE explanation (what to learn word-for-word)
-
Electrons fill the lowest-energy orbitals first (Aufbau principle).
-
In the Period 4 region of the periodic table, the 4s orbital is slightly lower in energy than the 3d orbitals in the neutral atom.
-
Therefore potassium (K) and calcium (Ca) put their next electrons into 4s before any electrons enter 3d.
-
When forming positive ions, electrons are removed from 4s before 3d.
That’s the wording most exam mark schemes love.
See it in the real configurations
Potassium, K (Z = 19)1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ → 4s fills before any 3d.
Calcium, Ca (Z = 20)… 3p⁶ 4s² → still no 3d yet.
Scandium, Sc (Z = 21)… 4s² 3d¹ → after 4s is full, electrons start entering 3d.
Ionisation tip: For transition metals, remove 4s electrons first.
Fe: [Ar] 4s² 3d⁶ → Fe²⁺ is [Ar] 3d⁶ (the two 4s electrons are gone).
The neat memory hook
“4s fills first, 4s leaves first.”
-
Fills first (K, Ca) because 4s is slightly lower in energy at that stage.
-
Leaves first (when ionising) because once electrons occupy both 4s and 3d and the atom becomes a cation, the effective energy ordering flips—the 4s electrons are higher in energy/less tightly held and go first.
Common exceptions you might hear about (extension)
Two famous configs you’ll meet later:
-
Chromium (Cr): [Ar] 3d⁵ 4s¹
-
Copper (Cu): [Ar] 3d¹⁰ 4s¹
They’re often taught as stability tweaks (half-filled or filled d subshells are especially stable). You don’t need to memorise these for every GCSE spec, but it’s nice context.
How to use this in exam answers
-
If asked “Why 4s before 3d?” write:
“In Period 4, the 4s orbital is slightly lower in energy than the 3d set, so 4s fills first. When forming ions, electrons are lost from 4s before 3d.” -
If asked to write configs for K, Ca, Sc, make sure you include 4s before any 3d and then start 3d at Sc.
45-second practice
-
Fill the blank: The order around Period 4 is … 3p → __ → __ → 4p.
-
Write the ending of the configuration for Sc (Z=21) and Ti (Z=22).
-
From which subshell do you remove electrons first to form Mn²⁺ from Mn?
Answers:
-
4s → 3d
-
Sc: … 4s² 3d¹ • Ti: … 4s² 3d²
-
4s (then 3d if more are needed)
What to remember from Part 9
-
Quote-ready: “4s fills before 3d; on ionisation, 4s empties before 3d.”
-
K, Ca end in 4s¹ / 4s²; 3d starts at Sc.
-
Keep exceptions (Cr, Cu) as extension only—don’t let them confuse the core rule.
Fast Ions Guide (score-easy marks)
Once you can write configurations, ions are mostly about removing from the right place.
Main-group quick wins
-
Sodium → Na⁺: loses the outer 3s¹
Na: 1s² 2s² 2p⁶ 3s¹ → Na⁺: 1s² 2s² 2p⁶ ([Ne]) -
Magnesium → Mg²⁺: loses 3s²
Mg: … 3s² → Mg²⁺: [Ne] -
Chlorine → Cl⁻: gains one to fill 3p
Cl: … 3p⁵ → Cl⁻: … 3p⁶ ([Ar])
Transition metals: the golden rule
Remove from 4s before 3d.
(Even though 4s filled first, those are the ones that come off first in ion formation.)
-
Iron, Fe (Z = 26)
Fe: [Ar] 4s² 3d⁶
Fe²⁺: [Ar] 3d⁶
Fe³⁺: [Ar] 3d⁵ -
Titanium, Ti (Z = 22)
Ti: [Ar] 4s² 3d²
Ti²⁺: [Ar] 3d²
Ti³⁺: [Ar] 3d¹
Exam tip: If a question says “Which electrons are removed first?” write:
“Electrons are removed from the outermost subshell first—4s before 3d in transition metals.”
Part 11 — Key Takeaways (one screen to revise)
-
Shells (n = 1,2,3…): higher n = further from nucleus + higher energy.
-
Subshells: s, p, d, f (number present equals n).
-
Orbitals: probability regions; max 2 electrons per orbital with opposite spins (Pauli).
-
Capacities: s2, p6, d10, f14 (rooms 1,3,5,7 → people 2,6,10,14).
-
Filling order (GCSE): 1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p.
-
Hund’s rule: in equal-energy orbitals, fill singly with parallel spins before pairing.
-
4s vs 3d: 4s fills first, 4s empties first.
-
First-20 shell model: often 2,8,8,2 (because 4s fills before 3d).
-
Shorthand: use nearest noble gas core, e.g. [Ne] 3s².
Part 12 — Practice Set (with answers)
A) Short answers
-
How many electrons fit in: (a) one p orbital, (b) the 2p subshell, (c) all 3d orbitals?
-
State Pauli’s Exclusion Principle in one sentence.
-
State Hund’s rule in one sentence.
-
Why does 4s appear before 3d in K and Ca?
B) Diagrams
-
Draw electrons-in-boxes for 2p in N (Z=7) and O (Z=8).
-
Draw electrons-in-boxes for 3p in P (Z=15).
C) Configurations (full + shorthand)
-
Al (Z=13) and Cl (Z=17).
-
K (Z=19), Ca (Z=20), Sc (Z=21).
-
Give configs for Fe²⁺ and Fe³⁺ starting from Fe.
Answers
A1. (a) 2 (b) 6 (c) 10
A2. Max two electrons per orbital, and they must have opposite spins.
A3. In equal-energy orbitals, electrons occupy singly with parallel spins before any pairing.
A4. In Period 4, 4s is slightly lower in energy than 3d, so it fills first; on ionisation, 4s empties first.
B5.
-
N (2p³):
\[↑] \[↑] \[↑] -
O (2p⁴):
\[↑↓] \[↑ ] \[↑ ]
B6.
-
P (3p³):
\[↑ ] \[↑ ] \[↑ ]
C7.
-
Al: 1s² 2s² 2p⁶ 3s² 3p¹ → [Ne] 3s² 3p¹
-
Cl: 1s² 2s² 2p⁶ 3s² 3p⁵ → [Ne] 3s² 3p⁵
C8.
-
K: … 3p⁶ 4s¹ → [Ar] 4s¹
-
Ca: … 3p⁶ 4s² → [Ar] 4s²
-
Sc: … 4s² 3d¹ → [Ar] 4s² 3d¹
C9.
-
Fe: [Ar] 4s² 3d⁶
-
Fe²⁺: [Ar] 3d⁶ (remove 4s²)
-
Fe³⁺: [Ar] 3d⁵ (remove one more from 3d)
Final checklist before you publish this series
-
Consistent terms (electronic configuration, subshell).
-
Correct filling order printed near examples.
-
A clear K/Ca/Sc trio to anchor 4s vs 3d.
-
A mini ions box: remove 4s before 3d.
Read more
Electrolysis Explained: Process, Examples & Applications
What Is an Atom? – Guide + Questions

Leave a Reply