Common GCSE Exam Mistakes (Acids, Bases and Salts)
Mistake 1: Confusing Strong and Concentrated Acids (GCSE Chemistry)
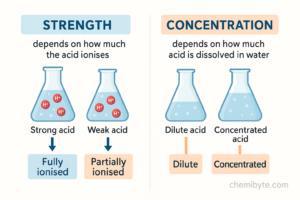
One of the most common mistakes in GCSE Chemistry is confusing the terms strong and concentrated when describing acids. Although these words sound similar, they mean completely different things and cannot be used interchangeably in exam answers.
A strong acid is an acid that fully ionises in water. This means that most or all of the acid molecules break apart to release hydrogen ions (H⁺). Examples of strong acids include hydrochloric acid and nitric acid.
A weak acid, such as ethanoic acid, only partially ionises in water. This means that fewer hydrogen ions are released, even if a lot of the acid is present.
On the other hand, concentration refers to how much acid is dissolved in water, not how much it ionises. A concentrated acid contains a large amount of acid per unit volume, while a dilute acid contains less.
Because strength and concentration describe different properties:
-
a strong acid can be dilute
-
a weak acid can be concentrated
For example, a small amount of hydrochloric acid dissolved in a large volume of water is still a strong acid, but it is dilute.
Why This Loses Marks in Exams
Students often write phrases like “a concentrated acid is strong”, which is incorrect. Examiners expect:
-
strong / weak → used for ionisation
-
concentrated / dilute → used for amount dissolved
Using the wrong term can cost marks even if the rest of the answer is correct.
How to Avoid This Mistake (GCSE Tip)
Before writing your answer, ask yourself:
-
Am I talking about how much the acid ionises? → use strong or weak
-
Am I talking about how much acid is present? → use concentrated or dilute
Mistake 2: GCSE Chemistry pH Scale Mistakes Students Make
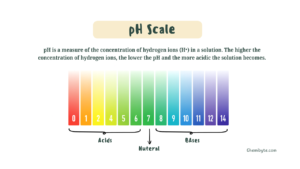
Another very common GCSE Chemistry mistake is misunderstanding how the pH scale works. Many students remember the numbers but do not fully understand what they mean.
The pH scale runs from 0 to 14:
-
pH 7 is neutral
-
pH values below 7 are acidic
-
pH values above 7 are alkaline
A frequent mistake is thinking that pH 0 is neutral or that pH 14 is only slightly alkaline. In reality, pH 0 represents a very strong acid, and pH 14 represents a very strong alkali.
Common Student Errors
Students often:
-
say pH 7 is acidic ❌
-
think pH 1 and pH 3 are almost the same ❌
-
forget that lower pH means more acidic, not less
At GCSE level, you do not need calculations, but you must correctly describe acidity and alkalinity using pH values.
Why This Loses Marks in Exams
Examiners expect students to:
-
correctly identify whether a substance is acidic, neutral, or alkaline
-
correctly compare two pH values
For example, if asked which solution is more acidic, the one with the lower pH must be chosen.
How to Avoid This Mistake (GCSE Tip)
Always remember:
-
Lower pH = more acidic
-
Higher pH = more alkaline
-
pH 7 = neutral
Writing these ideas clearly can earn easy marks in exams.
Mistake 3: Writing Incorrect or Unbalanced Chemical Equations (GCSE Chemistry)
One of the most common causes of lost marks in GCSE Chemistry exams is writing incorrect or unbalanced chemical equations. Even when students understand the reaction, mistakes in equations can cost easy marks.
A frequent error is forgetting to balance equations. In chemistry, the number of each type of atom must be the same on both sides of the equation. If an equation is not balanced, it is chemically incorrect.
Another common mistake is writing the wrong products. For example:
-
forgetting to include water in neutralisation reactions
-
forgetting carbon dioxide in acid–carbonate reactions
-
writing hydrogen gas in reactions where it is not produced
Students also often forget or misuse state symbols such as (s), (l), (g), and (aq). While not always required, state symbols are often expected in higher-mark questions.
Why This Loses Marks in Exams
Examiners mark equations very strictly. Even a small mistake, such as a missing coefficient or an incorrect product, can result in zero marks for the entire equation.
How to Avoid This Mistake (GCSE Tip)
Before moving on:
-
check that all atoms are balanced
-
check that the products match the reaction type
-
add state symbols if the question asks for them
Quick Reaction Reminders (GCSE)
-
Acid + metal → salt + hydrogen
-
Acid + base → salt + water
-
Acid + carbonate → salt + water + carbon dioxide
Mistake 4: Missing Key Scientific Keywords in GCSE Chemistry Answers
A very common reason students lose marks in GCSE Chemistry exams is not using the correct scientific keywords in written answers. Even when the idea is correct, marks can be lost if key terms are missing.
In topics such as acids, bases, and neutralisation, examiners expect specific words to be used. Writing vague explanations or everyday language instead of scientific terms often results in partial or no marks.
For example, in a neutralisation question, students may write that “the acid and alkali cancel each other out”. While this idea is partly correct, it does not include the scientific explanation that examiners are looking for.
Examiners usually expect keywords such as:
-
hydrogen ions (H⁺)
-
hydroxide ions (OH⁻)
-
neutralisation
-
salt
-
water
Without these terms, the answer may be considered incomplete.
Why This Loses Marks in Exams
GCSE Chemistry mark schemes are keyword-based. This means:
-
correct ideas without correct terms may not score full marks
-
explanations must show chemical understanding, not just general meaning
How to Avoid This Mistake (GCSE Tip)
When answering extended questions:
-
include ion names where relevant
-
use words like neutralisation, acid, alkali, and salt
-
avoid vague phrases such as “they react together” without explanation
Using the correct keywords can be the difference between 1 mark and full marks.
Mistake 5: Choosing the Wrong Method for Preparing Salts (GCSE Chemistry)
A common mistake in GCSE Chemistry is choosing the wrong method when answering questions about salt preparation. Students often remember the reactions but do not think carefully about which method is appropriate.
This mistake usually happens when students ignore whether a reactant is soluble or insoluble in water. For example, some students try to remove a soluble base by filtration, which is not possible because it dissolves in water. Others choose a metal that is below hydrogen in the reactivity series, which will not react with acids.
In salt preparation questions, examiners are not only testing whether you know the reactions, but whether you understand why a particular method is used.
Why This Loses Marks in Exams
Marks are often lost when:
-
an insoluble base is not filtered off correctly
-
a soluble base is treated as if it were insoluble
-
a metal below hydrogen is chosen to react with an acid
-
titration is ignored when it is clearly required
Even if the reaction is written correctly, using the wrong method can cost several marks.
How to Avoid This Mistake (GCSE Tip)
Before choosing a method, always ask:
-
Is the reactant soluble or insoluble?
-
Can excess reactant be removed by filtration?
-
Is the metal above hydrogen in the reactivity series?
Thinking about these points helps you choose the correct salt preparation method and score full marks.

this is so informative