Table of Contents
ToggleAlkali Metals Explained: Properties, Reactions, and Real-Life Uses
Have you ever wondered — what are alkali metals?
When you think about metals, names like iron, steel, or aluminium probably come to mind. These are strong, shiny materials we see in buildings, tools, and everyday objects.
But there’s another group of metals that are quite different – they’re soft, lightweight, and extremely reactive. These are the alkali metals, found in Group 1 of the periodic table.
In this blog, we’ll take a closer look at what makes alkali metals so unique. From their surprising reactions with water to their real-life uses in batteries and farming — this is your complete guide to understanding Group 1 elements in a simple, step-by-step way.
If you’re studying Chemistry or just curious about the periodic table, you’re in the right place.
Let’s get started.
What Are Alkali Metals?
Alkali metals are a group of highly reactive metals found in Group 1 of the periodic table. They include:
-
Lithium (Li)
-
Sodium (Na)
-
Potassium (K)
-
Rubidium (Rb)
-
Cesium (Cs)
-
Francium (Fr)
These metals all have one electron in their outermost shell, which makes them eager to react – especially with water and oxygen.
Why Are They Called Alkali Metals?
They’re called alkali metals because when they react with water, they form alkaline solutions – which means basic or high pH solutions. These reactions produce a metal hydroxide and hydrogen gas.
For example:
$$
\ce{2Na + 2H2O -> 2NaOH + H2 ^}
$$
In this reaction, sodium hydroxide (NaOH) is formed – a strong alkali.
Fun Fact:
Did you know?
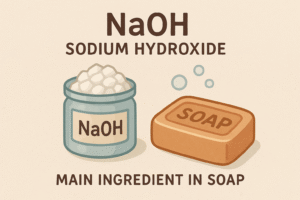
Sodium hydroxide (NaOH), made from alkali metals like sodium, is one of the main ingredients in soap.
It’s a strong alkali that helps turn fats and oils into soap in a process called saponification.
That’s why soaps are actually alkaline (basic) — all thanks to Group 1 metals!
Physical Properties of Alkali Metals
At first glance, alkali metals might not seem very “metal-like” at all. They’re actually quite different from typical metals like iron or copper.
Here are the key physical properties of alkali metals:
-
Softness: You can cut lithium, sodium, or potassium with a knife. The softness increases as you move down the group.
-
Low Density: Lithium, sodium, and potassium are less dense than water – they actually float.
-
Low Melting and Boiling Points: Compared to most metals, alkali metals melt and boil at relatively low temperatures.
-
Shiny When Cut: Freshly cut alkali metals have a silvery, shiny surface – but it quickly turns dull as it reacts with air.
-
Good Conductors: Like other metals, they conduct heat and electricity.
Fun Fact:
Did you know?
Potassium is so soft and reactive that it can actually catch fire when exposed to air — that’s why it’s stored under oil in labs!
Chemical Properties and Reactions of Alkali Metals
Alkali metals are extremely reactive, especially when they come into contact with water, oxygen, or halogens. Their reactions are fast, sometimes explosive and very predictable.
Here are the three main types of reactions they show:
1. Reaction with Water
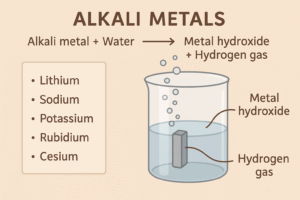
Alkali metals react vigorously with cold water to form:
-
A metal hydroxide (which is an alkali)
-
And hydrogen gas
The reaction gets more intense as you go down the group. For example:
\[
\ce{2Na + 2H2O -> 2NaOH + H2 ^}
\]
What you’d see in a lab:
-
The metal fizzes and floats
-
Heat is released
-
Sometimes, the gas ignites – especially with potassium!
2.Reaction with Oxygen (Air)
When alkali metals are exposed to air or oxygen, they quickly lose their shine and form metal oxides on the surface. This is why a freshly cut piece of sodium or potassium turns dull within seconds.
But not all alkali metals form the same type of oxide.
Here’s what happens:
| Element | Main Product Formed in Air | Type of Compound | Reaction Equation |
|---|---|---|---|
| Lithium | Lithium oxide (Li₂O) | Simple oxide | 4Li + O₂ → 2Li₂O |
| Sodium | Sodium oxide (Na₂O) and peroxide (Na₂O₂) | Mixture of oxide + peroxide | 4Na + O₂ → 2Na₂O 2Na + O₂ → Na₂O₂ |
| Potassium | Potassium superoxide (KO₂) | Superoxide | K + O₂ → KO₂ |
| Rubidium | Rubidium superoxide (RbO₂) | Superoxide | Rb + O₂ → RbO₂ |
| Cesium | Cesium superoxide (CsO₂) | Superoxide | Cs + O₂ → CsO₂ |
| Francium | Unknown – too reactive and radioactive | No observed reaction | Predicted to form superoxide or oxide, but never observed directly |
Why does the product change?
-
As the metals get more reactive down the group, they form stronger oxidizers like peroxides and superoxides.
-
Lithium (the smallest and least reactive) forms a simple oxide.
-
Sodium forms both oxide and peroxide.
-
Potassium, rubidium, and cesium form superoxides, which contain the O₂⁻ ion.
Classroom Tip: These reactions are usually slower and safer than reactions with water — but they still show how reactive alkali metals are with oxygen, especially as you go down the group.
3. Reaction with Halogens
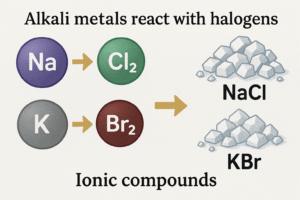
Alkali metals react quickly and easily with halogens (Group 17) like chlorine and bromine.
They form white crystalline salts called ionic compounds (e.g., sodium chloride – NaCl).
Example:
\[
\ce{2Na + Cl2 -> 2NaCl}
\]
Lab Safety Reminder
Because these reactions are exothermic and potentially dangerous, alkali metals are always handled in small amounts, with protective gear, and usually stored under oil.
Trends Down Group 1 – Alkali Metals
As you move down Group 1 in the periodic table — from lithium to francium — the properties of alkali metals follow clear, predictable trends. These trends help us understand how their reactivity and behavior change.
Here are the main patterns:
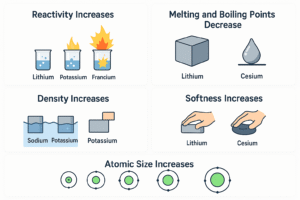
1. Reactivity Increases
The further down the group, the more reactive the metals become.
-
Lithium reacts slowly with water.
-
Potassium reacts quickly and catches fire.
-
Francium (if it could be seen) would explode instantly!
Why?
Because the outer electron is further from the nucleus in larger atoms, so it’s more easily lost — making reactions faster and more violent.
2. Melting and Boiling Points Decrease
-
Lithium has the highest melting point in Group 1.
-
Cesium melts just above room temperature!
This means alkali metals become softer and more heat-sensitive as you go down the group.
3. Density Increases (Mostly)
-
Lithium, sodium, and potassium are less dense than water.
-
Rubidium and cesium are more dense – they’ll sink in water.
Summary Table – Group 1 Trends
| Property | Trend Down Group 1 |
|---|---|
| Reactivity | Increases |
| Melting Point | Decreases |
| Boiling Point | Decreases |
| Density | Increases (with exceptions) |
| Softness | Increases (softer) |
| Atomic Size | Increases |
Why Are Alkali Metals So Reactive?
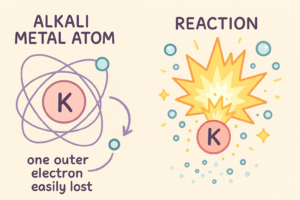
Alkali metals are some of the most reactive elements on the periodic table — but why?
It all comes down to their electron structure.
1. They Have Only One Outer Electron
Every alkali metal has just 1 electron in its outermost shell.
Atoms are always trying to become more stable, and the easiest way for Group 1 metals to do that is to lose that one electron.
When they lose it, they form +1 ions and become stable like noble gases.
2. The Outer Electron Is Loosely Held
As you move down the group, the atoms get bigger.
This means the outer electron is further away from the nucleus and is less tightly held.
So it’s easier to remove — making the metal more reactive.
3. Reactivity = Speed + Energy
Because it’s so easy for them to lose that electron, alkali metals:
-
React quickly
-
Release a lot of energy
-
Often cause fizzing, heat, or even small explosions
That’s why they’re stored under oil and handled with care in school labs.
[Insert image: Simple diagram showing alkali atom with 1 outer electron being lost]
📌 Alt text: Diagram showing alkali metal atom losing its outer electron to form a +1 ion
Safety Precautions with Alkali Metals
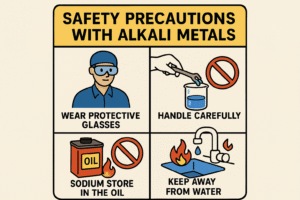
Because alkali metals are so reactive, especially with water and air, they must be handled very carefully — even in small amounts.
Here’s how scientists and students stay safe when working with Group 1 metals:
1. Stored Under Oil
Alkali metals are always kept under mineral oil or paraffin oil in containers.
This prevents them from reacting with moisture in the air or accidentally catching fire.
2. Use Safety Gear
In school labs or experiments:
-
Always wear goggles, gloves, and a lab coat
-
Use tongs or tweezers, not bare hands
-
Handle small pieces only
3. Keep Away from Water and Open Flames
Even a tiny drop of water can cause a reaction.
Make sure all equipment is dry and work is done away from flames or sinks.
4. Never Touch with Bare Hands
Alkali metals can burn skin or react with sweat, so direct contact should always be avoided.
Uses of Alkali Metals
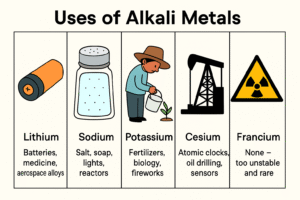
Even though alkali metals are too reactive to be used in their pure form, their compounds are extremely useful in everyday life, medicine, and technology.
Here’s how each one is used:
Lithium (Li)
-
Used in rechargeable batteries for phones, laptops, and electric cars
-
Helps treat bipolar disorder in small medical doses
-
Used in aerospace alloys to make lightweight aircraft parts
Sodium (Na)
-
Found in table salt (sodium chloride – NaCl)
-
Used in soap and detergent making
-
Sodium vapor used in street lights (yellow-orange glow)
-
Used in cooling systems in some nuclear reactors
Potassium (K)
-
Found in fertilizers – essential for plant growth
-
Plays a key role in the human body (nerve signals and muscle contraction)
-
Used in fireworks and explosives
Rubidium (Rb)
-
Used in special glasses and research
-
Plays a role in atomic clocks
Cesium (Cs)
-
Used in the most accurate atomic clocks on Earth
-
Helps in oil drilling as a component in drilling fluids
-
Used in radiation detectors and space tech
Francium (Fr)
-
No practical use
-
It’s too rare and radioactive to collect or study directly
Quick Table – Uses of Alkali Metals
| Element | Common Uses |
|---|---|
| Lithium | Batteries, medicine, aerospace alloys |
| Sodium | Salt, soap, lights, reactors |
| Potassium | Fertilizers, biology, fireworks |
| Rubidium | Glass making, research, atomic clocks |
| Cesium | Atomic clocks, oil drilling, sensors |
| Francium | None – too unstable and rare |
How Rubidium, Cesium, and Francium Are Different from Other Alkali Metals
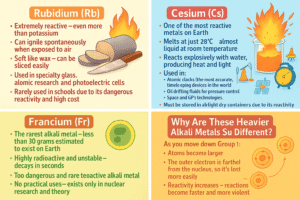
All alkali metals belong to Group 1 of the periodic table, and they all share some key properties — they are soft, have low melting points, and are highly reactive, especially with water and air.
But as we move down the group from lithium to francium, some major differences start to appear — especially with the bottom three: rubidium (Rb), cesium (Cs), and francium (Fr).
Here’s what sets them apart from the rest:
Rubidium (Rb)
-
Extremely reactive – even more than potassium
-
Can ignite spontaneously when exposed to air
-
Soft like wax – can be sliced easily
-
Used in specialty glass, atomic research, and photoelectric cells
-
Rarely used in schools due to its dangerous reactivity and high cost
Cesium (Cs)
-
One of the most reactive metals on Earth
-
Melts at just 28°C – almost liquid at room temperature
-
Reacts explosively with water, producing heat and light
-
Used in:
-
Atomic clocks (the most accurate timekeeping devices in the world)
-
Oil drilling fluids for pressure control
-
Space and GPS technologies
-
-
Must be stored in airtight, dry containers due to its reactivity
Francium (Fr)
-
The rarest alkali metal – less than 30 grams estimated to exist on Earth
-
Highly radioactive and unstable – decays in seconds
-
Predicted to be the most reactive alkali metal
-
Too dangerous and rare to study directly
-
No practical uses – exists only in nuclear research and theory
Why Are These Heavier Alkali Metals So Different?
As you move down Group 1:
-
Atoms become larger
-
The outer electron is farther from the nucleus, so it’s lost more easily
-
Reactivity increases – reactions become faster and more violent
-
Francium is also radioactive, making it unique even among Group 1 metals
Summary Tip:
In school, you’ll often experiment with lithium, sodium, and potassium, but not rubidium, cesium, or francium — because they’re too reactive, expensive, or radioactive to handle safely.
Practice Questions – Alkali Metals (GCSE Revision)
Test your understanding of everything you’ve learned so far! Try these practice questions — no pressure, just practice.
Q1:Which group do alkali metals belong to in the periodic table?
Q2:Why are alkali metals stored under oil?
Q3:What is formed when potassium reacts with water? Write a word equation.
Q4:Describe two trends in the physical properties of alkali metals as you go down Group 1.
Q5:Which alkali metal is used in rechargeable batteries?
Q6:Why does francium have no everyday uses?
Q7 (Challenge):Explain why potassium is more reactive than lithium, even though both have just one outer electron.
Key Takeaways – Alkali Metals
-
Alkali metals are found in Group 1 of the periodic table.
-
They are very reactive, especially with water and air.
-
Reactivity increases as you go down the group.
-
They form alkaline solutions and hydrogen gas when reacting with water.
-
Alkali metals are soft, have low melting points, and are stored under oil for safety.
-
Their compounds are widely used — in batteries, fertilizers, medicine, and clocks.
-
Francium is too rare and radioactive to have practical uses.
Glossary
Alkali Metals – A group of soft, reactive metals in Group 1 of the periodic table.
Reactive – Describes how easily a substance takes part in chemical reactions.
Group 1 – The first vertical column in the periodic table, containing alkali metals.
Metal Hydroxide – A basic compound formed when an alkali metal reacts with water (e.g. NaOH).
Hydrogen Gas – A colorless, flammable gas released during reactions with water.
Electron Shell – The layers around the nucleus where electrons orbit the atom.
Ionic Compound – A substance formed when atoms transfer electrons and stick together due to opposite charges.
Alkaline Solution – A basic (high pH) solution formed when a metal hydroxide dissolves in water.
Ask a Question or Keep Exploring!
Have a question about alkali metals? Curious about how other elements behave?
Leave a comment below
Read more
Read more Chemistry blogs here. keep learning.
World’s most expensive element Francium
Can we actually see atoms today