Chewable Metal? Indium – The Soft Surprise You Can Bend
Have you ever wondered that metal can be chewable? Indium metal might look like an ordinary silvery bar at first glance, but give it a gentle squeeze and you’ll see why chemists call it the “chewable” element. Softer than a pencil eraser and so malleable you can slice it with a fingernail, indium captivates scientists and hobbyists alike. In this post, we’ll uncover the crystal-structure secrets behind its extreme softness, watch its gallium alloy melt below room temperature, and learn how it coats glass to create mercury-free mirrors, all while exploring the real-world tech that depends on this surprisingly versatile metal.
Indium Metal
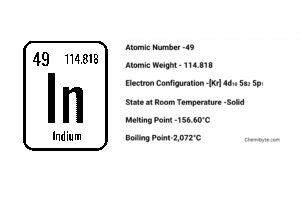
Indium (symbol In, atomic number 49) is a rare, silvery-white post-transition metal discovered in 1863 while analyzing zinc ore. It melts at just 156 °C, shows almost no corrosion in air, and finds its way into everything from phone touchscreens (as indium-tin oxide) to low-temperature solder and thermal interface pads. Despite these high-tech roles, a fresh bar feels more like soft lead than a typical “hard” metal—so soft you can slice it with a fingernail and hear a faint crackle when you bend it.
Why does it behave this way? The answer lies in its atomic bonding and crystal lattice:
| Factor | How It Keeps Indium Ultra-Soft |
|---|---|
| Weak metallic bonding | Only three valence electrons (5s² 5p¹) hold neighboring atoms together, so the bond strength is low. |
| Body-centered tetragonal lattice | This structure is loaded with “slip planes” that let atomic layers glide under very small forces. |
| Low shear modulus (~3.4 GPa) | Even gentle pressure lets dislocations move, making plastic deformation easy. |
| Large atomic radius | Widely spaced atoms reduce electrostatic attraction, further weakening the lattice. |
| Low melting point (156 °C) | A low melting point almost always pairs with low hardness—here, Mohs ≈ 1.2 (softer than a fingernail). |
| The “indium cry” | Bending breaks and reforms bonds in bursts, producing the tell-tale crackling sound—proof of how readily the lattice shears. |
Can You Really Cut Indium With Your Fingernail?
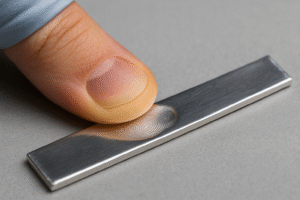
Yes, you really can cut indium with your fingernail.
Here’s why this metal behaves so differently from the hard ones you’re used to:
-
Very low hardness. Indium sits around 1.2 on the Mohs scale, while a fingernail is roughly 2.5 , so keratin easily scratches and slices the metal.
-
Weak metallic bonding. Only three loosely held valence electrons (5s² 5p¹) bond each atom to its neighbours, giving the lattice little strength.
-
Slip-friendly crystal lattice. Its body-centered tetragonal structure is packed with “easy-glide” planes, so atomic layers slide past one another under light pressure.
-
Low shear modulus (≈ 3–4 GPa). Minimal force is needed to start plastic deformation, letting a thumbnail act like a soft chisel.
-
Large atomic radius, low charge density. Widely spaced atoms feel weaker electrostatic attraction, further reducing overall hardness.
-
Low melting point (156 °C). A lower melting point usually tracks with weaker bonds and, therefore, greater softness.
Because all these factors work together, indium feels almost wax-like , soft enough for a single swipe of your nail to leave a clean, shiny groove.
Top Uses of Indium Metal in Touchscreens, Solder & Cooling
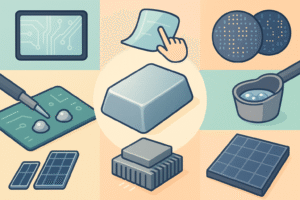
Indium’s unusual mix of softness, wetting ability, and electrical conductivity makes it indispensable in modern tech:
| Application | Why Indium Excels | Quick Fact |
|---|---|---|
| Indium-Tin Oxide (ITO) coatings for touchscreens & flat-panel displays | ITO is both transparent and conductive, so it registers touch without blocking light. | Used on virtually every capacitive smartphone screen. |
| Low-temperature solder alloys | Indium (or indium-lead/eutectic blends) melts well below 200 °C and wets metals easily, protecting heat-sensitive chips. | Common in aerospace and medical-device electronics. |
| Metal thermal-interface materials (TIMs) | Pure indium pads conduct heat ≈ 86 W m⁻¹ K⁻¹ and deform to fill microscopic gaps, slashing CPU or laser-diode temperatures. | Indium TIMs can cut junction temps by 10–20 °C vs. grease. (indiumcorporation) |
| CIGS thin-film solar cells | Copper-indium-gallium selenide absorbs sunlight so strongly that only a 1–2 µm layer is needed, enabling lightweight, flexible panels. | Lab efficiencies now top 22 %. |
Takeaway: From the glass on your phone to the solder under your laptop’s GPU, indium metal quietly keeps modern electronics bright, cool, and reliable.
Is Indium Metal Safe to Touch Or Even Chew?
In small, solid pieces, indium is considered low-toxicity, especially compared with mercury or lead. Casual handling or a quick “softness demo” poses little acute risk. Still, a few caveats matter:
- Inhalation is the real concern. Breathing indium oxide or indium-tin oxide (ITO) dust can scar lung tissue (“indium lung”). Such cases arise mainly in factory settings, not classrooms.
- Ingestion isn’t recommended. Data on swallowing metallic indium are limited, but any heavy-metal residue can accumulate—skip the snack.
- File shavings & wash hands. Fine particles enter the body more easily than solid strips; bag filings and clean up.
- Regulatory view. The U.S. National Institutes of Health and IARC rate some indium compounds (e.g., InP) as potentially carcinogenic when inhaled, but metallic indium itself is not classified as a human carcinogen. (PMC)
Bottom line: Touching indium bars for a classroom demo is generally safe with basic hygiene—just don’t inhale the dust or treat the metal as chewing gum.
Is indium Toxic?
In short, metallic indium is considered low-toxicity in solid form, but certain indium compounds and airborne dust can harm the lungs.
| Exposure route | What we know | Practical take-away |
|---|---|---|
| Handling solid bars, pellets, foil | Safety-data sheets list elemental indium as “relatively non-toxic” with few acute effects if no dust or fume is produced. | Touching or briefly “chewing” a clean strip is unlikely to cause immediate harm—just wash hands afterward. |
| Breathing indium dust or fume (e.g., indium-tin oxide, indium oxide) | Repeated inhalation can lead to “indium lung” (alveolar proteinosis, fibrosis); cases are documented in display-panel and ITO target factories. | Always control dust: work under fume hoods, wear respirators, and follow the NIOSH REL of 0.1 mg m⁻³ (8-h TWA). |
| Soluble indium salts or injected compounds | Animal studies show kidney and liver toxicity at high doses; some compounds are classified as possibly carcinogenic via inhalation. | These are lab-grade hazards, not an issue with solid metal demos, but require gloves and closed handling. |
Bottom line:
-
Solid elemental indium is one of the least toxic heavy metals for casual handling.
-
Indium dust, fumes, and certain chemical compounds can be dangerous to the lungs and should be controlled with proper ventilation and PPE.
Treat filings and vapours with respect, but a quick classroom “soft metal” demo with a solid bar is generally safe if you keep the area clean and wash up afterward.
Key Takeaways
-
Indium is uniquely soft. With a Mohs hardness of ~1.2, it’s one of the few metals your fingernail can scratch or even slice.
-
Liquid-metal alloys exist. Mix indium with gallium and the eutectic melts around 15 °C—turning solid metal to liquid in your hand.
-
Quiet tech workhorse. Indium’s transparency-plus-conductivity (as ITO), low-temp soldering, and high thermal conductivity keep modern electronics bright, cool, and reliable.
-
Generally low acute toxicity. Solid indium bars are safe to handle with basic hygiene, though indium dust and certain compounds do pose lung hazards.
-
Skip the snack. Demonstrations are fine, but filings shouldn’t be inhaled or ingested—bag them and wash your hands.
Author
I’m Aera RK, a chemist-turned-science-writer. At Chemibyte I break down chemistry topics into clear, bite-size posts for students and anyone who’s simply curious about how matter works.
Read more of my posts: Chemibyte

Amazing post. I didn’t know there’s a chewable metal that’s not highly toxic too. However, I’m curious about it’s price and abundance in nature.
indium is one of those rare metals that flies under the radar. While it’s not highly toxic like lead or mercury, it’s still not meant to be chewed like candy.
As for your question: indium is actually quite rare in nature. It’s usually found as a byproduct during zinc ore processing. Because of this, its supply is limited, which makes it relatively expensive. Prices can vary, but high-purity indium metal often costs hundreds of dollars per kilogram.