Introduction- How Did Scientists First Discover That Water Is Made of Hydrogen and Oxygen?
How scientists discovered water is made of hydrogen and oxygen is one of the most fascinating stories in chemistry history. Before this discovery, people believed water was a basic element. In our previous post on atomic models, we explored how scientists developed different ideas to explain what atoms are and how they behave. But here’s something interesting: even before those atomic models were proposed, scientists already knew that water is made of hydrogen and oxygen.
That discovery played a big role in shaping early atomic theories – especially Dalton’s atomic model. When John Dalton introduced his model in the early 1800s, he based it partly on the fact that water always breaks down into two parts hydrogen and one part oxygen.
So how did scientists figure this out in the first place?
How did they know water wasn’t just a basic element?
Let’s take a simple journey through the experiments and ideas that led to one of the most important discoveries in chemistry: water is H₂O.
What Did People Think Water Was Made Of?

Before modern science developed, people believed that water was a basic element one of the four fundamental substances in nature, along with earth, fire, and air. This idea came from ancient Greek philosophers like Empedocles and Aristotle, who thought everything in the world was made from a mix of these four elements.
No one had the tools or knowledge to test what water was really made of. It looked simple, clear, and essential, so it was treated as a pure substance.
But as science advanced in the 1700s, that idea began to change.
The Discovery of Hydrogen and Oxygen

In the late 1700s (18th century), science was starting to move away from old ideas like the “four elements” and toward something more experimental. Instead of guessing, scientists began testing. One area that caught their attention was gases.
At the time, air was seen as just… air. But researchers soon found that what we call “air” is actually made up of different types of gases, each with its own behavior.
Two of the most important discoveries during this time were:
-
Oxygen, discovered by Joseph Priestley in 1774
-
Hydrogen, discovered by Henry Cavendish in 1766
These gases didn’t behave like ordinary air at all.
-
Oxygen made flames burn brighter and longer
-
Hydrogen was light and flammable – it could explode when lit in the right conditions!
This was new and exciting. It meant that air wasn’t just one thing — it was a mixture, and each gas inside it could play a different role.
These discoveries set the stage for a new question:
If air is made of different gasses…
Could water also be made of more than one kind of particles?
The answer came soon after, in an experiment that changed everything.
Can Water Be Made From Gases? The First H₂ + O₂ Reaction
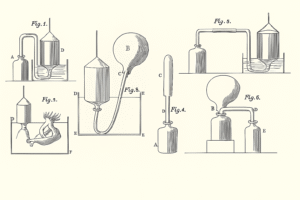
In the late 1700s, Henry Cavendish was experimenting with gases that were just being discovered. One of those gases was what he called “inflammable air”, today, we know it as hydrogen. This experiment was key to understanding how scientists discovered water is made of hydrogen and oxygen.
Cavendish mixed this hydrogen gas with oxygen, and then ignited the mixture.
The result was straightforward:
Water formed. Small droplets appeared on the container walls.
There were no byproducts or leftover gases just water. This observation suggested something new:
Water could be produced by combining hydrogen and oxygen.
At the time, this was surprising. Water had long been thought of as a basic substance , an “element” in the old sense. But Cavendish’s results hinted that water might actually be made from something else.
Later, Antoine Lavoisier — working independently — confirmed this idea. He repeated the experiment and gave a clear explanation:
When hydrogen gas burns in oxygen, it forms water.
This was one of the first chemical reactions ever carefully measured, and it played a key role in shifting scientific opinion. Water wasn’t an element. It was a compound made from two different gases.
Electrolysis of Water: How Scientists Split H₂O with Electricity
After scientists saw that hydrogen and oxygen could combine to form water, the next question was:
Can we break water back into hydrogen and oxygen?
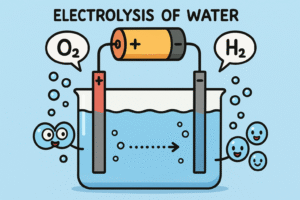
The answer came with the invention of a method called electrolysis.
In the early 1800s, scientists including William Nicholson and Anthony Carlisle discovered that if you passed an electric current through water, it would begin to break down.
Here’s how it works:
-
You place two electrodes (wires or metal rods) into water.
-
You connect them to a battery.
-
Bubbles start to form at both electrodes.
But not equally – and that part is key.
-
One side produces hydrogen gas, and the other side produces oxygen gas.
-
The hydrogen side always has twice as many bubbles as the oxygen side.
This confirmed something important:
Water is made of two parts hydrogen and one part oxygen – in a 2:1 ratio.
That’s why the chemical formula is H₂O.
Electrolysis gave scientists a way to see the parts of water separately, and measure them.
No matter how many times the experiment was repeated, the results were always the same.
This wasn’t a guess anymore – it was measurable, repeatable, and scientific. The process of electrolysis played a major role in how scientists discovered water is made of hydrogen and oxygen.
Why Scientists Say Water Is Made of Hydrogen and Oxygen (H₂O)
By the early 1800s, scientists had strong evidence that water could be made from hydrogen and oxygen, and also broken apart into those same gases.
But there was still one important question:
Why do you always get twice as much hydrogen as oxygen?
Why not equal parts? Or some other ratio?
This clue came from two things:
1. Volume Measurements from Electrolysis
When water was split using electricity, the amount of gas collected was always consistent:
-
For every 2 volumes of hydrogen, there was 1 volume of oxygen
This showed that water must contain twice as many hydrogen particles as oxygen particles.
2. Dalton’s Atomic Theory
Around the same time, John Dalton introduced his atomic theory. He suggested that:
-
All matter is made of atoms
-
Atoms of different elements combine in simple, whole-number ratios
Water became a perfect example.
To explain the 2:1 gas ratio, Dalton proposed that one water molecule is made of:
-
2 hydrogen atoms (H)
-
1 oxygen atom (O)
That’s why the formula for water is written as H₂O – not H₂O₂ or HO.
This simple ratio helped scientists understand how chemical formulas work, and how atoms come together to form compounds.
Making Water by Burning Hydrogen
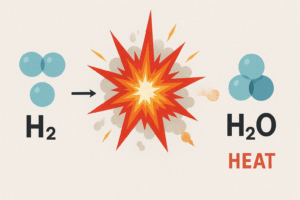
Even today, you can see the formation of water from hydrogen and oxygen in action — in a very dramatic way.
If you ignite hydrogen gas in the presence of oxygen, you’ll get a small explosion and the only product left behind is water vapor.
Hydrogen + Oxygen → Water + Heat
This is the reverse of electrolysis. Instead of using electricity to break water down, we’re releasing energy by bringing hydrogen and oxygen together.
This chemical reaction is used in some real-world applications:
-
Rocket engines often use liquid hydrogen and liquid oxygen as fuel. When they react, they produce a powerful burst of energy and water is the only byproduct.
-
In science labs, this reaction is demonstrated to show that water can be formed from two gases, proving its chemical structure.
So whether you’re splitting water or creating it, the ratio never changes:
Two parts hydrogen to one part oxygen – H₂O.
Glossary
-
Atom – The smallest unit of an element, made of protons, neutrons, and electrons
-
Molecule – Two or more atoms chemically bonded together
-
Hydrogen (H) – A light, flammable gas and the most abundant element in the universe
-
Oxygen (O) – A gas essential for breathing and burning; supports combustion
-
Electrolysis – A process that uses electricity to split a compound into its elements
-
Chemical Reaction – A process where substances are changed into new substances
-
Compound – A substance made of two or more elements joined in a fixed ratio
-
H₂O – The chemical formula for water, showing it contains two hydrogen atoms and one oxygen atom
-
Covalent Bond – A strong chemical bond formed when atoms share electrons
-
Lavoisier – The chemist who explained water formation and helped develop modern chemical naming
Key Takeaways
-
Scientists once believed water was a simple element, but experiments showed it’s a compound.
-
Cavendish discovered that burning hydrogen in oxygen forms water.
-
Electrolysis confirmed that water splits into 2 parts hydrogen and 1 part oxygen.
-
That’s why we write water’s formula as H₂O.
-
This discovery helped build modern atomic theory and chemical science.

