Introduction
Have you ever looked at a piece of jewellery and wondered how it got that flawless gold coating? It is not paint, and it is definitely not done with a tiny brush. The secret lies in a process called electrolysis.
Electrolysis is where electricity steps in and makes chemical reactions happen that normally would not. With the right setup, it can tear water apart into hydrogen and oxygen, pull pure metals out of stubborn ores, or coat one metal with another so smoothly it looks like magic.
What makes it even more fascinating is that this process has a mirror image. While electrolysis uses electricity to push reactions forward, some reactions can actually create electricity on their own. That is the principle behind batteries and fuel cells, which are everyday proof that chemistry and electricity are deeply connected.
In this blog, we will explore what electrolysis really is, how it works once you strip away the jargon, and the ways it shapes both science and daily life.
What is Electrolysis?
Electrolysis is the use of electricity to make a chemical reaction happen when it would not occur on its own. The name tells the story clearly: electro means electricity, and lysis means to split apart. In simple terms, electrolysis is all about using electrical energy to break substances down.
Think of water, for example. On its own, it looks calm and stable, but when electricity is applied it suddenly splits into hydrogen and oxygen. The same idea works for many other compounds. Salty water can be broken into chlorine, hydrogen, and sodium hydroxide. Aluminium oxide, when melted, gives pure aluminium and oxygen. Even gold can be deposited onto jewellery by moving its ions with electricity.
These examples show the heart of electrolysis. Electricity pushes ions to move, and at the electrodes they either gain or lose electrons. This changes them from charged particles into new atoms or molecules, and that change is what gives us useful products.
Perfect — examples make it much easier for beginners to really picture what is happening. I’ll keep the same clear and natural flow, and add a few simple examples right into the explanation.
How Electrolysis Works
Think of electrolysis as electricity convincing particles to change. On their own, most substances are stable and do not split apart. But once electricity is applied, the particles begin to move and react in new ways.
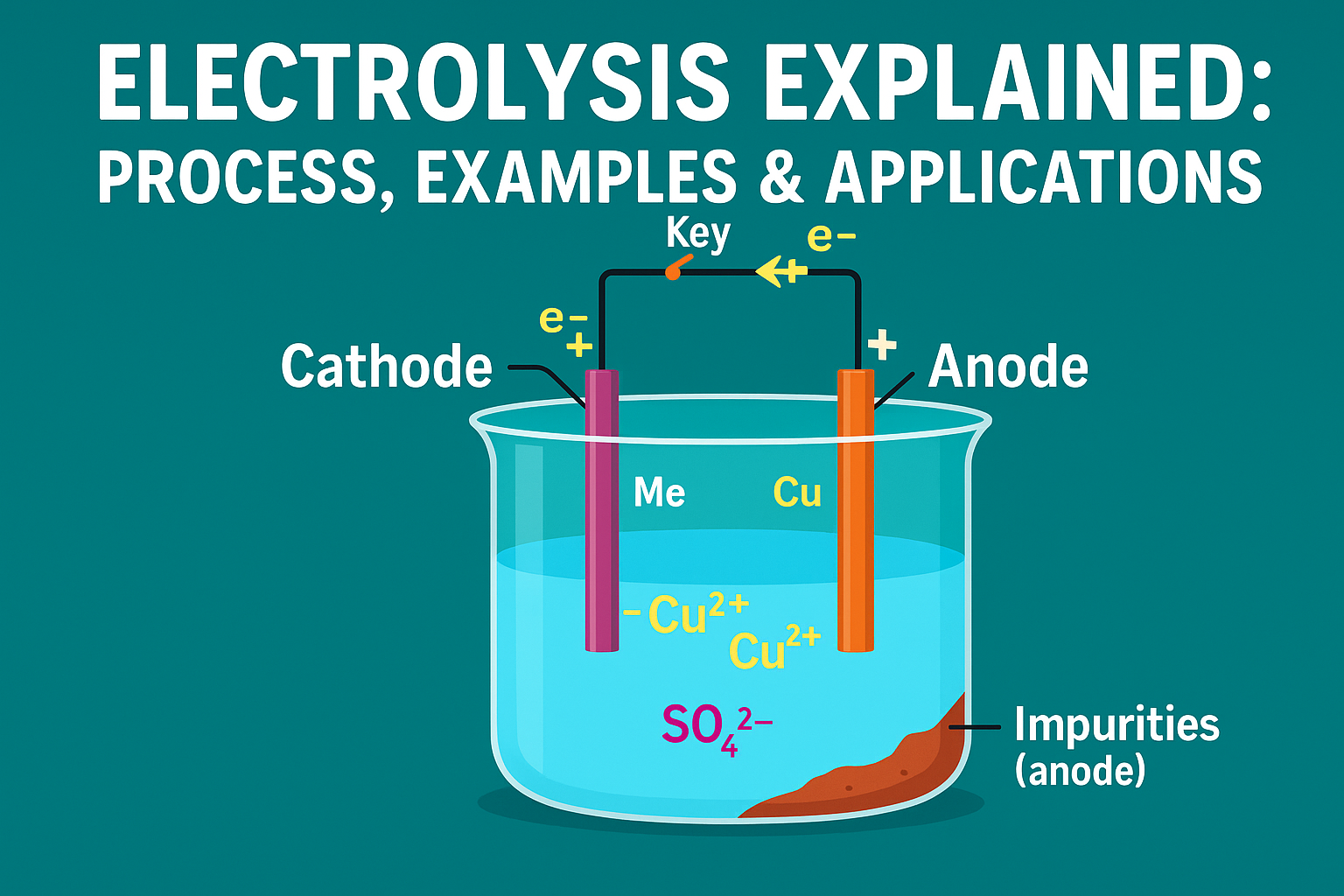
When the current passes through, positive ions drift toward the negative side, called the cathode. There they gain electrons and become neutral atoms. For example, in copper refining, copper ions in the solution travel to the cathode and turn into pure copper metal, which slowly forms a solid layer.
On the other side, negative ions move toward the positive electrode, called the anode. Here they give away their extra electrons. In the electrolysis of salt water, chloride ions release electrons at the anode and join together to form chlorine gas, which escapes as bubbles.
Sometimes electrolysis works by breaking things apart. A common school experiment is splitting water into hydrogen and oxygen. Bubbles of hydrogen form at one electrode, and oxygen forms at the other, always in a two-to-one ratio because of the formula H₂O.
Overall reaction:
2H₂O → 2H₂ + O₂
Other times electrolysis builds things up. A well-known example is electroplating, where electricity makes a thin layer of one metal coat another. Gold or silver can be plated onto jewellery to give it a shiny and valuable finish without using solid pieces of those metals.
So electrolysis can either break a compound down into simpler substances or build up a new material on a surface. In both cases, the key is that electricity moves the ions, and the electrodes give them a place to gain or lose electrons. That simple idea powers many of the examples we see in science labs and industries.
How Do We Perform Electrolysis?
To really understand electrolysis, it helps to see how the process is carried out in practice. A simple electrolysis experiment needs three key parts:
-
A power supply (usually a battery or DC source)
-
Two electrodes (often made of inert materials such as graphite or platinum)
-
An electrolyte (a substance that contains free ions, either molten or dissolved in water)
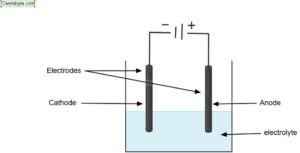
The power supply provides direct current, which is essential because it pushes ions in one fixed direction. If the current kept switching sides, as in alternating current, the ions would never settle and no useful products would form.
The electrodes are solid conductors, usually metal rods or graphite, placed into the substance. They connect the liquid or molten material to the power supply so that electricity can flow through.
The electrolyte is the substance that will be broken down. It must contain free ions that can move, which is why we use either molten ionic compounds or solutions where salts are dissolved in water. Without free-moving ions, electricity cannot pass and no reaction can occur.
Once everything is connected, the current flows through the electrolyte. Ions move, chemical changes take place, and new substances appear at the surfaces of the electrodes. This simple setup, used in classrooms with small batteries, is the same principle that industries rely on when producing metals, gases, or chemicals on a large scale.
The Essentials Needed for Electrolysis
Now that we know how the electrodes behave during electrolysis, it’s worth looking more closely at the three essential components themselves. Each plays a unique role in making electrolysis possible:
1. Power Supply in Electrolysis
Why do we need a Power Supply?
Electrolysis is all about forcing a chemical reaction to happen using electricity.
-
Many ionic substances won’t break apart on their own.
-
The battery or DC power pack gives energy to push electrons into or out of ions at the electrodes.
-
Without an external power supply, the reaction wouldn’t start.
Think of the power supply as the engine that drives the entire process.
Why Direct Current (DC) Works in electrolysis
In direct current (DC):
-
One terminal is always positive (+) → connected to the anode.
-
One terminal is always negative (–) → connected to the cathode.
-
This creates fixed electrodes:
-
Cathode (–): attracts positive ions (cations).
-
Anode (+): attracts negative ions (anions).
-
Because the poles never switch, ions know exactly where to move:
-
Positive ions always travel toward the cathode.
-
Negative ions always travel toward the anode.
This steady movement allows useful chemical reactions to happen.
Why Alternating Current (AC) Doesn’t Work in electrolysis
In alternating current (AC):
-
The polarity of the terminals switches back and forth (50–60 times per second).
-
That means the cathode and anode keep swapping roles.
-
Ions would move one way, then instantly be pulled the opposite way.
The result:
-
No clear movement of ions.
-
No stable chemical products.
-
Just confusion in the solution!
That’s why electrolysis only works with DC.
Beginner’s Analogy
Imagine DC electricity as a one-way road: cars (ions) always know where to go no confusion.
Now imagine AC as a road that switches direction every second: cars would stop, turn, crash, and never reach their destination. That’s why only DC can make electrolysis work.
So in short:
-
-
-
DC provides fixed electrodes → ions move in the right direction.
-
AC confuses ions → no useful reaction.
-
Industries need massive DC power → but it’s costly, so cheap electricity = cheaper products.
-
-
2. Electrodes in Electrolysis
What Are Electrodes?
-
Electrodes are solid conductors (usually rods or plates) placed in the electrolyte.
-
They connect the power supply to the solution, so electricity can enter and leave.
-
More importantly, they provide a surface where ions gain or lose electrons.
In simple words: electrodes are the meeting point where electricity and chemistry interact.
The Two Electrodes
-
Cathode (negative electrode)
-
Connected to the negative terminal of the DC power supply.
-
Attracts positive ions (cations).
-
Ions gain electrons here (reduction happens).
-
-
Anode (positive electrode)
-
Connected to the positive terminal of the DC power supply.
-
Attracts negative ions (anions).
-
Ions lose electrons here (oxidation happens).
-
Types of Electrodes
1. Inert Electrodes (don’t react)
-
Examples: Graphite (carbon), Platinum.
-
They only act as a pathway for electrons — they don’t take part in the chemistry themselves.
-
Used when we want products to come only from the electrolyte.
Example:
-
Electrolysis of water (H₂O → H₂ + O₂).
-
Hydrogen forms at the cathode, oxygen forms at the anode.
-
The electrodes themselves stay unchanged.
2. Reactive Electrodes (do react)
-
Example: Copper electrodes.
-
These electrodes themselves take part in the reaction.
Example: Copper purification
-
Anode (impure copper): Copper atoms dissolve into solution as Cu²⁺ ions.
-
Cathode (pure copper): Cu²⁺ ions from the solution gain electrons and deposit as pure copper.
-
The impure anode gradually gets smaller, while the cathode becomes a thick sheet of pure copper.
This process is extremely important for making high-quality copper for electrical wiring, since pure copper is a much better conductor.
Why Electrodes Matter
-
They decide where the reaction happens → oxidation at the anode, reduction at the cathode.
-
They can also influence what products form:
-
Inert electrodes → products depend only on the electrolyte.
-
Reactive electrodes → products may come partly from the electrodes themselves.
-
So, electrodes aren’t just “metal sticks.” They’re active players that guide, control, and sometimes even join the reaction.
Beginner’s Analogy
Think of electrolysis like a football game:
-
The power supply is the referee that controls the flow.
-
The electrolyte is the field where the players (ions) move around.
-
The electrodes are the goalposts → they decide where the action happens and where the points (reactions) are scored.
In summary:
-
Electrodes provide the surface for electron transfer.
-
Inert electrodes don’t react (just carry current).
-
Reactive electrodes can dissolve or deposit material.
-
They control both the location and sometimes the products of electrolysis.
3.Electrolyte in Electrolysis
What is an Electrolyte?
The electrolyte is the substance that gets broken down during electrolysis.
-
It must contain free ions that can move around.
-
These ions carry the electric charge through the liquid.
-
Without free-moving ions, electrolysis cannot happen.
In short: the electrolyte is the “fuel” of the process.
Types of Electrolytes
1. Molten Ionic Compounds
-
If we heat an ionic compound until it melts, the solid lattice breaks, and the ions are free to move.
-
Example: Molten sodium chloride (NaCl)
-
Na⁺ ions move to the cathode → gain electrons → form sodium metal.
-
Cl⁻ ions move to the anode → lose electrons → form chlorine gas.
-
This method is used in industry to extract reactive metals like sodium, aluminium, magnesium, etc.
2. Aqueous Solutions (ionic compounds dissolved in water)
-
When an ionic compound dissolves in water, its ions separate and can move freely.
-
Example: Copper sulfate (CuSO₄ solution)
-
Contains Cu²⁺ and SO₄²⁻ ions.
-
Also contains H⁺ and OH⁻ ions from water.
-
Because water also provides ions, the products can sometimes be less predictable.
-
At the cathode, you might get hydrogen gas (from H⁺) or metal (from Cu²⁺) depending on conditions.
-
At the anode, you might get oxygen (from OH⁻) or a non-metal (like Cl₂ if chloride is present).
That’s why electrolysis in water-based solutions can be more complicated.
3. Acids and Alkalis
-
Acids release H⁺ ions in water.
-
At the cathode → H⁺ ions gain electrons → hydrogen gas forms.
-
-
Alkalis (bases) release OH⁻ ions in water.
-
At the anode → OH⁻ ions lose electrons → oxygen gas forms.
-
This is why school demonstrations of electrolysis often use:
-
Dilute sulfuric acid (H₂SO₄) → produces hydrogen and oxygen.
-
Sodium hydroxide (NaOH) → also produces hydrogen and oxygen.
Beginner’s Analogy
Think of the electrolyte as the “crowd of players” in the game of electrolysis:
-
The power supply gives them energy.
-
The electrodes tell them where to go.
-
The electrolyte decides which players (ions) are in the game, and therefore what products are made.
In summary:
-
Electrolyte = source of ions.
-
Molten salts → simple, predictable products (metal + non-metal).
-
Aqueous solutions → more complex because water joins in.
-
Acids/alkalis → give hydrogen and oxygen, useful for experiments.
Why Do We Use Molten Substances In Electrolysis Instead of Aqueous Solutions?
Electrolysis is all about moving ions, but the medium (molten vs aqueous) makes a huge difference to the products we get. Let’s carefully unpack this.
1. Purity of Ions in Molten Compounds
When we melt an ionic compound, only the ions of that compound are present.
-
Example: Molten NaCl contains just Na⁺ and Cl⁻ ions.
-
This means at the electrodes, only sodium and chlorine are formed.
-
Result: The products are simple, clear, and pure.
That’s why industries love molten electrolysis — they know exactly what they’ll get.
2. Interference of Water in Aqueous Solutions
When an ionic compound is dissolved in water, we don’t just get the compound’s ions. We also get ions from water itself:
H2O⇌H++OH−H₂O ⇌ H⁺ + OH⁻
So in an aqueous solution of NaCl, we actually have four ions floating around:
-
Na⁺, Cl⁻ (from NaCl)
-
H⁺, OH⁻ (from water)
At the electrodes, these ions “compete” to be discharged.
-
At the cathode (–): H⁺ ions usually get reduced instead of Na⁺, because hydrogen is easier to discharge than sodium.
-
At the anode (+): Cl⁻ ions may be discharged, but if they are too dilute, OH⁻ from water may produce oxygen instead.
This makes the products less predictable compared to molten electrolysis.
3. Energy Considerations
Sometimes aqueous electrolysis is actually cheaper in the lab because it avoids the huge amount of energy needed to melt a compound. For example:
-
Melting Al₂O₃ requires extremely high temperatures. That’s why industries add cryolite to lower the melting point.
-
For simpler demonstrations, aqueous solutions are fine because they conduct electricity easily at room temperature.
So the choice often depends on whether you need pure products (industry → molten) or just a demonstration (school labs → aqueous).
4. Industrial Needs
-
Molten electrolysis: Used in large-scale production of metals (like aluminium and sodium) and chlorine, because industries want pure products.
-
Aqueous electrolysis: Used for making chemicals like hydrogen, oxygen, and sodium hydroxide (from brine).
Examples Side by Side
-
Molten NaCl
-
Products: Sodium (at cathode), Chlorine (at anode)
-
Pure, predictable, but requires very high temperature
-
-
Aqueous NaCl (Brine)
-
Products: Hydrogen (at cathode), Chlorine (at anode), Sodium hydroxide (in solution)
-
Mixed products, cheaper to run, used in chemical industry
-
Summary
-
Molten electrolysis = pure products, predictable, energy-heavy
-
Aqueous electrolysis = cheaper, easier in labs, but products are mixed due to water ions
What Happens at the Electrodes – Half-Equations Explained
So far, we know the setup: electricity flows in, electrodes are placed in the electrolyte, and ions move. But what exactly happens when those ions reach the electrodes?
That’s where half-equations come in.
What is a Half-Equation?
-
A half-equation shows the chemical change happening at one electrode only.
-
It explains whether ions gain electrons (reduction) or lose electrons (oxidation).
-
When you combine the half-equations from both electrodes, you get the overall reaction.
Easy rule to remember:
-
Cathode (–): Reduction (gain of electrons).
-
Anode (+): Oxidation (loss of electrons).
Step-by-Step: Writing a Half-Equation
-
Find the ion moving to the electrode.
-
Decide what happens: gain electrons (reduction) or lose electrons (oxidation).
-
Balance the charges by adding the right number of electrons (e⁻).
-
Balance the atoms on both sides.
Example 1: Electrolysis of Molten Sodium Chloride (NaCl)
-
At the Cathode (–):
Na++e−→NaNa⁺ + e⁻ → Na -
At the Anode (+):
2Cl−→Cl2+2e−2Cl⁻ → Cl₂ + 2e⁻
Products: sodium metal at the cathode, chlorine gas at the anode.
Example 2: Electrolysis of Water (with Acid)
-
At the Cathode (–):
2H++2e−→H22H⁺ + 2e⁻ → H₂ -
At the Anode (+):
4OH−→O2+2H2O+4e−4OH⁻ → O₂ + 2H₂O + 4e⁻
Products: hydrogen gas and oxygen gas.
Example 3: Copper Refining
-
At the Anode (+, impure copper):
Cu→Cu2++2e−Cu → Cu²⁺ + 2e⁻ -
At the Cathode (–, pure copper):
Cu2++2e−→CuCu²⁺ + 2e⁻ → Cu
Result: impure copper dissolves, pure copper builds up — key in making wires.
Why Half-Equations Matter
-
They show the flow of electrons clearly.
-
They help you balance chemical reactions.
-
They are heavily tested in school chemistry exams (GCSE, IGCSE, O/L, A/L).
Electrolysis of Water
One of the simplest experiments in school labs is splitting water into hydrogen and oxygen using electrolysis. This shows students that water is not just a liquid, but actually made of different atoms that can be separated with electricity.
The Setup
-
Electrolyte: Pure water does not conduct electricity well. So we don’t use pure water as electrolyte. We add a little dilute sulfuric acid (H₂SO₄) or sodium hydroxide (NaOH) to provide ions.
-
Electrodes: Inert electrodes like graphite or platinum.
-
Power Supply: Direct current (DC), so we have a fixed cathode (–) and anode (+).
What Happens at Each Electrode
-
At the Cathode (–): Hydrogen ions (H⁺) gain electrons and form hydrogen gas.
2H++2e−→H22H⁺ + 2e⁻ → H₂ -
At the Anode (+): Hydroxide ions (OH⁻) lose electrons and form oxygen gas.
4OH−→O2+2H2O+4e−4OH⁻ → O₂ + 2H₂O + 4e⁻
Products: Hydrogen gas at the cathode, Oxygen gas at the anode.
Observations in the Lab
-
Bubbles of hydrogen gas collect at the cathode.
-
Bubbles of oxygen gas collect at the anode.
-
The ratio of gas volumes is roughly 2:1 (twice as much hydrogen as oxygen), because water has the formula H₂O.
How to confirm the gases evolved are oxygen and hydrogen?
Real-World Importance
-
Hydrogen from electrolysis is being explored as a clean fuel in the future.
-
Oxygen is used in industry for welding, hospitals, and water treatment.
-
This simple experiment connects directly to green energy research today.
Factors Affecting Electrolysis
Electrolysis does not always give the same products. The results depend on several important factors that control which ions are discharged at the electrodes. Let’s look at them one by one.
1. Type of Electrolyte (Molten vs Aqueous)
Molten Electrolytes
-
In a molten ionic compound, only the ions of that compound are present.
-
The products are straightforward because there is no competition from water.
-
Example: Molten sodium chloride (NaCl):
-
At the cathode (–): Na⁺ ions gain electrons to form sodium metal.
-
At the anode (+): Cl⁻ ions lose electrons to form chlorine gas.
-
This always produces the metal and the non-metal of the salt.
Aqueous Electrolytes
-
When an ionic compound is dissolved in water, the situation becomes more complex.
-
Water itself ionizes into H⁺ and OH⁻ ions, which compete with the ions of the dissolved salt.
-
Example: Aqueous copper sulfate (CuSO₄ solution):
-
At the cathode (–): Cu²⁺ competes with H⁺. Copper is usually discharged as metal.
-
At the anode (+): OH⁻ competes with SO₄²⁻. Oxygen is usually released.
-
In aqueous solutions, the products depend on both the electrolyte and the reactivity of the ions present.
2. Type of Electrodes (Inert vs Reactive)
Inert Electrodes (Graphite, Platinum)
-
Do not take part in the reaction.
-
The products come entirely from the electrolyte.
-
Example: Electrolysis of water using graphite electrodes produces hydrogen and oxygen.
Reactive Electrodes (e.g., Copper)
-
The electrodes themselves participate in the reaction.
-
This changes the products of electrolysis.
-
Example: Purification of copper:
-
At the anode (impure copper): copper atoms lose electrons and dissolve as Cu²⁺ ions.
-
At the cathode (pure copper sheet): Cu²⁺ ions gain electrons and are deposited as pure copper.
-
Here, the choice of electrode directly influences the products.
3. Position of Ions in the Reactivity Series
When multiple ions compete for discharge, the reactivity series helps decide the outcome.
At the Cathode (–):
-
If a metal ion competes with hydrogen ions (H⁺):
-
A less reactive metal (such as copper or silver) will be deposited as metal.
-
If the metal is very reactive (such as sodium, potassium, or calcium), hydrogen gas will be released instead.
-
At the Anode (+):
-
If halide ions (Cl⁻, Br⁻, I⁻) are present, they are usually discharged to form halogen gases.
-
If no halide ions are present, hydroxide ions (OH⁻) from water are discharged, producing oxygen gas.
In short:
-
At the cathode, the less reactive species is discharged.
-
At the anode, halides are discharged first; otherwise oxygen forms.
This section shows that the products of electrolysis are not random. They depend on the electrolyte used, the type of electrodes chosen, and the position of ions in the reactivity series.
Factors Affecting Electrolysis
Electrolysis may look like a simple process, pass current, collect gases or metals, and you’re done. But in reality, the products formed during electrolysis depend on several key factors. These factors influence which ions are discharged at the electrodes, how fast the reaction occurs, and even whether the process is economical for industry.
Let’s break them down one by one.
1. Type of Electrolyte (Molten vs Aqueous)
The electrolyte provides the ions needed for electrolysis. But the state of the electrolyte (molten or aqueous) can completely change the products.
-
Molten Electrolytes:
-
Only the ions from the compound itself are present.
-
Products are predictable, and there’s no interference from water.
-
Example: Molten sodium chloride (NaCl) → sodium metal at cathode, chlorine gas at anode.
-
-
Aqueous Electrolytes:
-
Water itself splits into H⁺ and OH⁻ ions.
-
These extra ions compete with the compound’s ions at the electrodes.
-
Example: Aqueous CuSO₄ contains Cu²⁺, SO₄²⁻, H⁺, and OH⁻.
-
At cathode: Copper (Cu²⁺) is discharged because it is less reactive than hydrogen.
-
At anode: Oxygen is released from OH⁻, not sulfate ions.
-
-
Why it matters: In molten salts, the outcome is clear and pure. In aqueous solutions, you need to consider competition between ions.
2. Nature of the Electrodes (Inert vs Reactive)
Electrodes are not just passive wires — they decide how the reaction proceeds.
-
Inert Electrodes (Graphite, Platinum)
-
Do not take part in the chemical reaction.
-
Products come only from the electrolyte.
-
Example: Electrolysis of water → hydrogen (cathode) and oxygen (anode).
-
-
Reactive Electrodes (e.g., Copper, Silver)
-
Electrodes themselves are involved in the reaction.
-
Example: Copper Refining
-
Anode (impure copper) → dissolves into solution as Cu²⁺.
-
Cathode (pure copper sheet) → Cu²⁺ ions deposit as pure copper.
-
Result: Pure copper builds up, impurities fall away.
-
-
Why it matters: Choice of electrode can change the product completely. Using copper vs graphite gives very different results.
3. Position of Ions in the Reactivity Series
When more than one cation or anion is present, the reactivity series decides which gets discharged.
-
At the Cathode (–)
-
Less reactive metals (e.g., copper, silver) are deposited as metal.
-
Very reactive metals (e.g., sodium, potassium, calcium) are not discharged; instead, hydrogen gas is produced.
-
-
At the Anode (+)
-
Halide ions (Cl⁻, Br⁻, I⁻) are discharged first to form halogen gases.
-
If halides are absent, OH⁻ from water is discharged, giving oxygen gas.
-
Why it matters: This rule ensures predictable outcomes in mixed electrolytes.
4. Concentration of Ions
When two ions can be discharged, concentration tips the balance.
-
Example: Aqueous NaCl (brine):
-
At high concentration → chlorine is released at the anode.
-
At low concentration → oxygen may be released instead.
-
Why it matters: Industrial brine electrolysis is carefully controlled by concentration to produce chlorine, hydrogen, and sodium hydroxide reliably.
5. Voltage / Current Supplied
The amount of voltage applied controls which ions are discharged:
-
Low Voltage: Only the expected ions (e.g., H⁺ and OH⁻ in water) are discharged.
-
High Voltage: Forces less-favoured ions to react, sometimes producing unexpected products.
Example:
-
Water electrolysis with normal voltage → hydrogen + oxygen.
-
At very high voltage → ozone (O₃) may form instead of just oxygen.
Why it matters: Voltage determines efficiency, cost, and safety of industrial electrolysis.
6. Temperature and Safety
-
High Temperatures: Needed for molten electrolysis (e.g., Al₂O₃ requires >900 °C). Cryolite is added in industry to lower the melting point.
-
Toxic Products: Some gases like chlorine are poisonous and require fume hoods or sealed equipment.
Why it matters: Without correct safety and temperature control, electrolysis can be dangerous or uneconomical.
Applications of Electrolysis
Electrolysis is not just a classroom experiment; it is a vital process in modern industry and everyday life. Here are the main applications explained in a little more detail:
1. Extraction of Metals
Some metals are so reactive that they cannot be extracted from their ores by heating with carbon. For example, aluminium, sodium, magnesium, and calcium form very stable compounds.
In these cases, the only way to obtain the metal is to use electrolysis of molten compounds.
-
Example: Aluminium is extracted from molten aluminium oxide (Al₂O₃) in the Hall–Héroult process. The ore is melted with cryolite (to reduce melting point), and when current passes, aluminium collects at the cathode while oxygen forms at the anode.
-
Why important? Without electrolysis, modern industries like aviation, construction, and packaging (which rely heavily on aluminium) would not exist.
2. Purification of Metals
Metals like copper must be extremely pure to be used in electrical wiring. Even small impurities reduce conductivity.
-
Process: In copper purification, the impure copper block acts as the anode, while a thin sheet of pure copper acts as the cathode. When electricity passes, copper atoms from the anode dissolve into solution as Cu²⁺ ions and then deposit onto the cathode as pure copper metal.
-
Why important? This ensures the copper used in wires, circuits, and motors is 99.99% pure, making them efficient and safe.
3. Electroplating
Electrolysis can deposit a thin layer of one metal on another surface. This process, called electroplating, improves appearance, prevents rusting, and sometimes improves conductivity.
-
Examples:
-
Silver plating jewellery to make cheaper metals look valuable.
-
Gold plating tiny electronic parts so they resist corrosion and carry current more effectively.
-
Chrome plating car parts for a shiny finish and rust protection.
-
-
Why important? It saves cost (only a thin coat of expensive metal is needed) while also improving durability and looks.
4. Manufacture of Chemicals
One of the biggest industrial uses of electrolysis is the production of chemicals from brine (concentrated NaCl solution).
-
Products:
-
Chlorine gas (Cl₂): used in making PVC plastics, disinfectants, and bleach.
-
Hydrogen gas (H₂): used in making margarine and as a clean energy source.
-
Sodium hydroxide (NaOH): used in soap, detergents, and paper production.
-
-
Why important? The “chlor-alkali industry” based on this process provides raw materials for hundreds of everyday products.
5. Production of Hydrogen and Oxygen
Electrolysis of water gives two very useful gases.
-
Hydrogen:
-
Clean energy fuel that produces only water when burned.
-
Being developed for hydrogen-powered vehicles and renewable energy storage.
-
-
Oxygen:
-
Essential in hospitals for patients with breathing difficulties.
-
Used in welding and cutting metals.
-
Plays a role in water treatment to improve quality.
-
-
Why important? As the world shifts towards green energy, electrolysis is becoming a key way to produce hydrogen fuel without polluting the environment.
Interesting Facts About Electrolysis
Electrolysis may look like a simple classroom experiment, but it connects to some fascinating real-world science.
-
Electrolysis is energy-hungry
-
The extraction of aluminium by electrolysis uses so much electricity that aluminium plants are usually built near hydroelectric power stations. Countries like Canada, Iceland, and Norway are global leaders in aluminium production for this reason.
-
-
It is behind the shine of jewellery and coins
-
Electroplating uses electrolysis to coat objects with a thin layer of precious metal like gold or silver. This process makes jewellery more attractive and protects coins and cutlery from corrosion.
-
-
Fuel of the future
-
Hydrogen gas from electrolysis of water is being explored as a clean alternative to fossil fuels. If powered by renewable energy, this process could play a major role in building a sustainable energy future.
-
-
Reverse Electrolysis exists
-
In electrolysis, we use electricity to force chemical reactions. But in some processes, the reaction itself produces electricity. This is sometimes called “reverse electrolysis” — and it is the principle behind batteries and fuel cells.
-
For example, in a hydrogen fuel cell, hydrogen and oxygen combine to produce water while releasing electricity.
-
This is the opposite direction of water electrolysis, and shows how chemistry and electricity are deeply connected.
-
-
First discovered in the 1800s
-
The word “electrolysis” was first coined by Michael Faraday in 1834. His famous “laws of electrolysis” still form the foundation of our understanding today.
-
Looking Ahead
Electrolysis is more than just splitting compounds; it is a process that powers industries, shapes our daily lives, and even points toward a renewable future.
In this guide, we explored:
-
The essentials of electrolysis
-
What happens at electrodes (half-equations)
-
Factors that affect the process
-
Applications in industry and daily life
Conclusion
Electrolysis is more than just splitting compounds; it is a process that powers industries, shapes our daily lives, and even points toward a renewable future.
In this guide, we explored:
-
The essentials of electrolysis
-
What happens at electrodes (half-equations)
-
Factors that affect the process
-
Applications in industry and daily life
Electrolysis remains one of the most important bridges between chemistry and electricity a discovery from the 19th century that continues to power the 21st.
read more chemistry blogs
Can we actually see atoms today