Introduction – Law of Definite Proportions
Why does water always behave like water, no matter where it comes from? Why do compounds like carbon dioxide and copper carbonate always form in the same way?
Before scientists understood atoms, they were already noticing something strange but consistent. When elements combine to form compounds, they do so in exact, fixed ratios by mass.
This observation became one of the most important ideas in chemistry. It is now known as the Law of Definite Proportions.
In this article, you will learn:
-
What the law of definite proportions really means
-
How Joseph Proust discovered it through experiment
-
Why this law changed the way we understand matter
-
Real-world examples that prove it in action
What Is the Law of Definite Proportions?
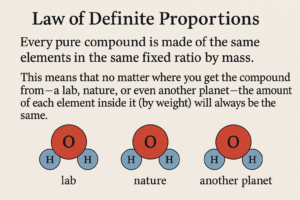
The Law of Definite Proportions says:
Every pure compound is made of the same elements in the same fixed ratio by mass.
This means that no matter where you get the compound from a lab, nature, or even another planet the amount of each element inside it (by weight) will always be the same.
Example 1: Water (H₂O)
Water is made of 2 hydrogen atoms and 1 oxygen atom.
But hydrogen and oxygen don’t weigh the same. Here’s how much they weigh:
Mass Ratio of Elements in Water (H₂O)
| Element | Number of Atoms | Mass of Each Atom (units) | Total Mass (units) |
|---|---|---|---|
| Hydrogen (H) | 2 | 1 | 2 |
| Oxygen (O) | 1 | 16 | 16 |
Final Mass Ratio
| Hydrogen : Oxygen |
|---|
| 2 : 16 → 1 : 8 |
For every 1 gram of hydrogen, there are 8 grams of oxygen in water.
Example 2: Carbon Dioxide (CO₂)
Mass Ratio of Elements in Carbon Dioxide (CO₂)
| Element | Number of Atoms | Mass of Each Atom (units) | Total Mass (units) |
|---|---|---|---|
| Carbon (C) | 1 | 12 | 12 |
| Oxygen (O) | 2 | 16 | 32 |
Final Mass Ratio
| Carbon : Oxygen |
|---|
| 12 : 32 → 3 : 8 |
For every 3 grams of carbon, there are 8 grams of oxygen in carbon dioxide.
How Did Scientists Discover the Law of Definite Proportions?
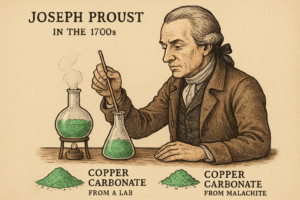
In the late 1700s, scientists were trying to understand how substances form. They couldn’t see atoms, because there were no microscopes strong enough at the time. But they had one powerful tool — a balance. They could measure mass very carefully.
A French chemist named Joseph Proust wanted to know:
Do elements always combine in the same way, or can the amounts change?
To find out, he studied a compound called copper carbonate. It’s a green solid made of copper, carbon, and oxygen. Proust collected copper carbonate from two sources:
-
One sample was made in a lab
-
Another was found in nature, from a mineral
He broke both samples down using chemical methods and measured the mass of each element inside.
What he found was surprising.
The Result
No matter where the copper carbonate came from, the mass of copper, carbon, and oxygen was always the same.
The elements were always combined in the same ratio by mass.
This proved that copper carbonate was not just a mixture, but a true compound. Its composition didn’t change.
What Proust Discovered
In 1804, Proust shared his findings and gave chemistry one of its first big rules:
A pure compound always contains the same elements in the same proportion by mass.
This became known as the Law of Definite Proportions.
Why Was This Discovery Important?
At the time, not everyone believed that elements always combined in fixed amounts. Some scientists thought you could mix elements in any ratio to make a compound.
But Proust’s discovery proved that this was not true. His careful experiments showed that compounds always had the same makeup. That changed how scientists thought about matter.
Here’s why it was a big deal:
1. It Showed That Compounds Are Predictable
Compounds like water, carbon dioxide, and copper carbonate are not random mixtures.
They are made of elements that always join in fixed, exact amounts.
This meant that scientists could now start to:
-
Measure compounds more precisely
-
Write correct formulas for them
-
Predict how much of each element would be in a reaction
2. It Helped Prove That Atoms Are Real
At that time, atoms were still just an idea — no one had seen them.
But if elements always combine in the same mass ratio, it made sense to think that:
-
Tiny particles (atoms) were joining together in set numbers
-
These particles were real and had different weights
This led to a stronger belief in atoms and set the stage for the next big step in chemistry Dalton’s Atomic Theory
Dalton’s Atomic Theory and Simple Ratios
After Joseph Proust showed that elements always combine in fixed mass ratios, another scientist took the idea even further.
His name was John Dalton. He was an English chemist who wanted to explain why these mass ratios happened.
Since no one could see atoms at the time, Dalton used logic and data from experiments including Proust’s to build a new idea.
What Did Dalton Believe?
In 1808, Dalton introduced a theory called Atomic Theory. He believed:
-
All matter is made of tiny particles called atoms
-
Atoms of each element are unique in mass and properties
-
When elements form compounds, their atoms join in simple, whole-number ratios
This theory helped explain the Law of Definite Proportions.
Linking Atoms to Mass Ratios
Let’s take water (H₂O) again as an example:
-
Water has 2 hydrogen atoms and 1 oxygen atom
-
Each hydrogen atom weighs 1 unit
-
The oxygen atom weighs 16 units
So the total mass = 2 + 16 = 18 units
This matches the mass ratio we saw before:
2 hydrogen units : 16 oxygen units → simplified to 1 : 8
Dalton said this wasn’t just coincidence.
It was because atoms were joining in fixed numbers, like 2 hydrogen + 1 oxygen.
Why This Was a Big Step
Dalton’s theory gave chemistry a structure:
-
It explained why mass ratios are fixed
-
It helped scientists write formulas like H₂O and CO₂
-
It turned chemistry into a more exact and measurable science
Real-Life Examples of Fixed Ratios in Compounds
Once scientists accepted the Law of Definite Proportions, they started seeing the same fixed patterns in many other compounds.
Let’s look at some easy examples that show how elements always combine in set mass ratios.
Example 1: Water (H₂O)
Water is made of 2 hydrogen atoms and 1 oxygen atom.
| Element | Atoms | Mass of Each Atom | Total Mass |
|---|---|---|---|
| Hydrogen | 2 | 1 unit | 2 units |
| Oxygen | 1 | 16 units | 16 units |
Mass ratio = 2 : 16 = 1 : 8
No matter where the water comes from — river, lab, or bottle — this ratio is always the same.
Example 2: Carbon Dioxide (CO₂)
Carbon dioxide is made of 1 carbon atom and 2 oxygen atoms.
| Element | Atoms | Mass of Each Atom | Total Mass |
|---|---|---|---|
| Carbon | 1 | 12 units | 12 units |
| Oxygen | 2 | 16 units | 32 units |
Mass ratio = 12 : 32 = 3 : 8
Whether it comes from your breath, burning wood, or dry ice, the ratio stays the same.
Example 3: Copper Carbonate (CuCO₃)
This is the compound Proust studied in his famous experiment. It contains:
-
Copper (Cu)
-
Carbon (C)
-
Oxygen (O)
In every sample he tested from a lab or from nature the mass of each element was always in the same ratio.
This showed it was a true compound, not a mixture.
These examples helped scientists understand that compounds are not made randomly.
The elements inside them always follow a pattern.
Are There Any Exceptions of Definite Proportions
Yes, in most cases, the Law of Definite Proportions is true. But there are a few exceptions where compounds do not always have the exact same mass ratio of elements.
These are more advanced topics, but here are two simple examples to help you understand.
1. Non-Stoichiometric Compounds
Some compounds can have a variable composition. This means the ratio of elements is not always the same.
A good example is wüstite (a mineral made mostly of iron and oxygen).
Its chemical formula is close to FeO, but the amount of iron and oxygen inside can change slightly. That means the mass ratio is not always fixed.
This happens because of small gaps or extra atoms in the structure of the compound.
2. Isotopes
Elements like carbon and oxygen have different versions of their atoms called isotopes. Isotopes have the same chemical behavior, but their mass is slightly different.
So if one sample of water has slightly different isotopes than another, the mass ratio might very slightly change — but the chemical formula stays the same.
These exceptions are rare and usually do not affect the basic understanding of chemical compounds.
For most everyday compounds like water and carbon dioxide, the Law of Definite Proportions still holds true.
Important Questions on the Law of Definite Proportions
| Law of Definite Proportions Questions (Left Side) | Law of Definite Proportions Questions (Right Side) |
|---|---|
| 1. What does the Law of Definite Proportions say about compounds? A. Compounds can have any ratio B. Elements combine randomly C. Each compound has a fixed mass ratio D. Elements change when heated |
2. According to the Law of Definite Proportions, water always has a hydrogen to oxygen mass ratio of 1:8. If you have 4 g of hydrogen, how much oxygen is needed? A. 8 g B. 16 g C. 32 g D. 64 g |
| 3. Who discovered the Law of Definite Proportions? A. John Dalton B. Antoine Lavoisier C. Joseph Proust D. Niels Bohr |
4. Why is the Law of Definite Proportions important in chemistry? A. It proves atoms exist B. It explains radioactivity C. It breaks the atomic theory D. It is used in nuclear reactions |
| 5. Which compound follows the Law of Definite Proportions? A. A sample that changes color B. A mixture of salt and sand C. Water with a 1:8 hydrogen to oxygen ratio D. A solution that evaporates quickly |
6. A compound has 3 g of carbon and 8 g of oxygen. According to the Law of Definite Proportions, how much oxygen is needed for 9 g of carbon? A. 16 g B. 24 g C. 6 g D. 9 g |
| 7. The Law of Definite Proportions proves that… A. Atoms join in fixed ratios B. Energy is always conserved C. Gases expand when heated D. Elements can be separated easily |
8. Does the compound wüstite (FeO) always follow the Law of Definite Proportions? A. Yes B. No C. Sometimes D. Only when heated |
| 9. Two samples of copper carbonate were tested. Both had the same mass ratio of copper, carbon, and oxygen. What does this confirm? A. The samples are different compounds B. The Law of Definite Proportions is false C. Both samples follow the Law D. The copper was pure |
10. According to the Law of Definite Proportions, a compound’s mass ratio… A. Depends on pressure B. Stays the same in all samples C. Changes with size D. Changes with state (solid/liquid) |
Answer Key:
| Q | Answer | Q | Answer |
|---|---|---|---|
| 1 | C | 2 | C |
| 3 | C | 4 | A |
| 5 | C | 6 | B |
| 7 | A | 8 | B |
| 9 | C | 10 | B |
Glossary: Key Terms
CompoundA substance made from two or more elements that are chemically bonded in a fixed ratio.
| Term | Definition |
|---|---|
| Compound | A substance made from two or more elements that are chemically bonded in a fixed ratio. |
| Law of Definite Proportions | A chemistry law stating that a given compound always contains the same proportion of elements by mass. |
| Mass Ratio | The comparison of the mass of one element to another in a compound. |
| Element | A pure substance that cannot be broken down into simpler substances by chemical means. |
| Joseph Proust | A French chemist who proposed the Law of Definite Proportions in the late 1700s. |
| Hydrogen to Oxygen Ratio (1:8) | The mass ratio of hydrogen to oxygen in water (H₂O); for every 1 g of hydrogen, there are 8 g of oxygen. |
| Wüstite (FeO) | A compound of iron and oxygen, often used in examples of compounds that may not follow the law strictly due to non-stoichiometric behavior. |
Have Questions?
If you have any questions about the Law of Definite Proportions or want help understanding related chemistry topics, feel free to leave a comment below we’re here to help!
Or explore more beginner-friendly chemistry lessons on our blog:
View All Chemibyte Articles →

