Introduction:States of Matter
Everything around us air, water, metal, or even plastic is made of something called matter. But matter doesn’t always look or act the same way. Why? Because it can exist in different forms called “states.”
This is one of the first big ideas you learn in chemistry: matter can be solid, liquid, or gas. Each state has different properties, and these differences come from how the tiny particles inside behave.
 In this GCSE Chemistry guide, we’ll explore:
In this GCSE Chemistry guide, we’ll explore:
-
What the three states of matter are
-
How particles move and interact in solids, liquids, and gases
-
How substances change from one state to another (like melting, freezing, or boiling)
-
Why things behave the way they do at room temperature
We’ll also use diagrams and real examples to help you understand and remember the key ideas whether you’re revising for an exam or just curious about how the world works.
The 3 States of Matter Explained (GCSE Chemistry)
Before we jump into solids, liquids, and gases, let’s take a moment to ask a simple question:
What is matter?
Matter is anything that takes up space and has mass.
That means everything around you the desk you’re leaning on, the water in your bottle, even the air you’re breathing it’s all matter.
Now here’s something interesting. Matter might seem smooth and solid to us, but scientists discovered that it’s not actually continuous.
Instead, all matter is made up of tiny particles so small that we can’t see them with our eyes.
But how do we know that?
Let’s go back in time for a moment. For a long time, people thought that matter was just one solid piece — like a block of wood or a lump of clay. But as science developed, researchers noticed some strange behaviours.
 For example:
For example:
-
Gases can expand to fill a container
-
Substances can change state when heated or cooled
-
Liquids can mix and spread on their own (like perfume in air)
None of these things made sense unless something else was going on.
Eventually, scientists proposed a new idea — one that changed chemistry forever.
They suggested that matter is actually made up of tiny, moving particles and that there are spaces between them.
This became known as the particle theory of matter.
What Does Particle Theory Tell Us?
Let’s break it down into simple points:
-
All matter is made of tiny particles
-
These particles are always moving
-
Even in solids, the particles vibrate in place
-
There are spaces between the particles – more in gases, less in solids
-
The more energy the particles have (like when something is heated), the faster they move
-
The way particles are arranged and how they move explains why matter behaves differently in each state
So even something as solid as a table is full of particles constantly vibrating, with tiny gaps between them.
That’s the starting point. And once you understand this, it becomes much easier to explain why solids are firm, why liquids flow, and why gases fill up space.
Let’s take a closer look at those three states now.
What Are the 3 States of Matter?
Now that we know matter is made of tiny particles, let’s explore what happens when those particles are arranged in different ways.
This brings us to one of the most important ideas in chemistry:
Matter exists in different forms, called states.
At GCSE level, we focus on the three main states of matter:
-
Solids
-
Liquids
-
Gases
Each state has its own properties and those properties come from how the particles are arranged and how they move.
Let’s look at them one by one.
Solids
 In solids, the particles are packed closely together in a fixed, regular pattern.
In solids, the particles are packed closely together in a fixed, regular pattern.
They don’t move from place to place – they simply vibrate in position.
That’s why solids have:
-
A definite shape
-
A fixed volume
-
And they usually feel hard or firm
You can’t pour a solid, and it won’t change shape to fit its container – because the particles are locked in place.
Examples of solids:
Ice, salt, glass, wood, metals
Liquids
 In liquids, the particles are still close together, but they’re not in a fixed pattern.
In liquids, the particles are still close together, but they’re not in a fixed pattern.
They can slide past each other, which is why liquids can flow.
Liquids don’t have a fixed shape they take the shape of their container. But they do have a fixed volume.
Examples of liquids:
Water, milk, oil, alcohol
Gases
 In gases, the particles are far apart and move very quickly in all directions.
In gases, the particles are far apart and move very quickly in all directions.
They have much more energy than solids or liquids.
This explains why gases:
-
Have no fixed shape
-
Have no fixed volume
-
Can expand to fill any space
Gases are easy to compress because of all the empty space between the particles.
Examples of gases:
Oxygen, carbon dioxide, helium, steam
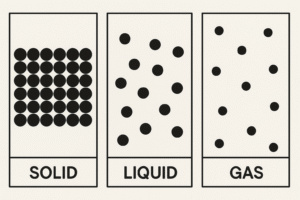
Particle Diagrams of Solids, Liquids and Gases
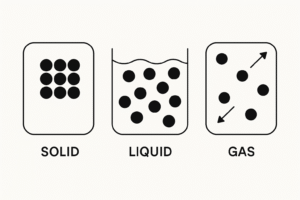
To help us understand the differences between solids, liquids, and gases, scientists use particle diagrams. These are simple drawings that show how particles are arranged in each state of matter.
Even though we can’t see particles with our eyes, these diagrams give us a helpful model of what’s happening inside the material.
Let’s look at how each one is drawn – and what it tells us.
Solid – Fixed and Tightly Packed
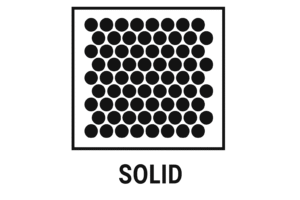
In Solids
-
Arrangement: Particles are tightly packed in a regular pattern
-
Movement: They don’t move around — they just vibrate in place
This is why:
-
Solids keep their shape
-
They can’t be compressed
-
They stay firm and fixed
Even though they seem completely still, the particles in a solid are always vibrating. The colder the solid, the slower the vibrations. The warmer it gets, the faster they vibrate — until the solid melts.
Diagram Description:
Imagine neat rows of small circles tightly packed together — no gaps, no movement between them.
Liquid – Close, But Able to Flow
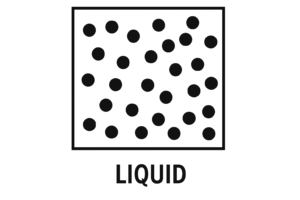
In Liquids
-
Arrangement: Particles are close together but not in a set pattern
-
Movement: They can slide past each other
This is why:
-
Liquids can flow
-
They take the shape of their container
-
But they still have a fixed volume (they don’t shrink or expand easily)
Liquids have more energy than solids, so the particles move around more freely but not enough to break completely apart.
Diagram Description:
Particles are close, but not in lines. They’re scattered and slightly touching, imagine a loose crowd gently moving past each other.
Gas – Spread Out and Fast Moving
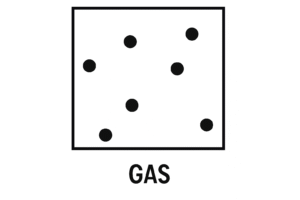
In Gases
-
Arrangement: Particles are far apart and randomly spread out
-
Movement: They move very quickly in all directions
This is why:
-
Gases fill the entire space they’re in
-
They can be compressed easily
-
They don’t have a fixed shape or volume
The particles in a gas have lots of energy and almost no attraction to each other. That’s why they zoom around, bouncing off walls and each other.
Diagram Description:
Particles are spaced out with lots of gaps in between. They’re spread across the whole area, not touching, and pointing in all directions.
How Particles Behave in Solids, Liquids & Gases
We’ve looked at how solids, liquids, and gases appear in particle diagrams. But why do they behave so differently?
It all depends on how their particles are arranged and how they move.
-
In solids, particles are tightly packed and only vibrate in place → this is why solids hold a fixed shape.
-
In liquids, particles are close together but can slide past each other → this is why liquids can flow and take the shape of a container.
-
In gases, particles are far apart and move quickly in all directions → this is why gases spread out and don’t have a fixed shape or volume.
Now, what controls this behavior?
It comes down to two main things:
-
Kinetic energy – how much energy the particles have (how fast they move)
-
Forces of attraction – how strongly the particles pull toward each other
Let’s look at both, one at a time.
What Is Kinetic Energy?
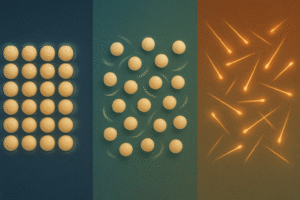 Kinetic energy is the energy of movement.
Kinetic energy is the energy of movement.
Particles are always moving — and the more energy they have, the faster they move.
-
In solids, particles have low kinetic energy. They just vibrate in place.
-
In liquids, particles have more kinetic energy. They can slide and flow.
-
In gases, particles have high kinetic energy. They move fast and spread out.
When we heat something, we’re giving its particles more kinetic energy that’s why heating causes changes of state.
What Are Forces of Attraction?
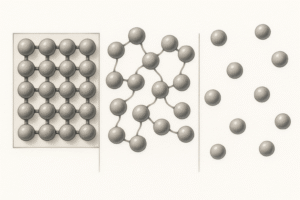 Particles are not just moving around randomly. They also pull on each other a bit like invisible magnets. These are called intermolecular forces or forces of attraction.
Particles are not just moving around randomly. They also pull on each other a bit like invisible magnets. These are called intermolecular forces or forces of attraction.
-
In solids, the forces are very strong – that’s why particles stay locked together in a fixed shape.
-
In liquids, the forces are weaker – strong enough to keep particles close, but not in place.
-
In gases, the forces are very weak or almost zero – particles are free to fly apart.
How Energy and Attraction Work Together
These two factors explain everything:
-
Low energy + strong attraction → particles stay locked together → solid
-
Medium energy + weaker attraction → particles move and slide → liquid
-
High energy + very weak attraction → particles move freely and spread → gas
So next time you see steam rising or ice melting, remember it’s all about tiny particles moving differently, based on their energy and how much they’re pulling on each other.
That’s exactly what happens when you heat a substance:
-
A solid melts and becomes a liquid
-
A liquid boils and becomes a gas
Why does this happen?
Because the particles gain energy as you heat them.
With more energy, they move faster and eventually break free from the forces that were holding them together.
That’s how a block of ice becomes water, and then turns into steam all because the particles are changing how they move and how close they are to each other.
Changes of State – Melting, Freezing, Boiling, and More
Now that we understand how particles move and interact, it’s time to look at how substances change from one state to another.
These are called changes of state and they happen when a substance is heated or cooled. The important thing to remember is:
These are physical changes. The substance stays the same only its form changes. ( No chemical changes occur)
Let’s look at each one.
Melting
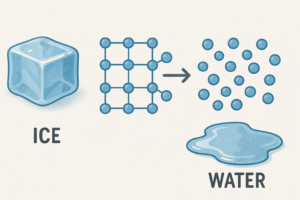 Solid → Liquid
Solid → Liquid
When a solid is heated, its particles gain energy. Eventually, they start to move more and more until they can break free from their fixed positions. That’s when the solid melts into a liquid.
-
The temperature where this happens is called the melting point.
-
The particles are still close, but now they can slide past each other.
Example: Ice melting into water
Freezing
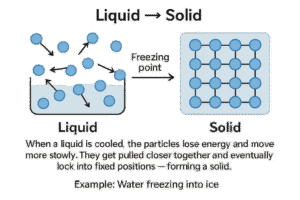 Liquid → Solid
Liquid → Solid
When a liquid is cooled, the particles lose energy and move more slowly. They get pulled closer together and eventually lock into fixed positions — forming a solid.
-
This happens at the freezing point, which is the same temperature as the melting point (just in reverse).
Example: Water freezing into ice
Boiling
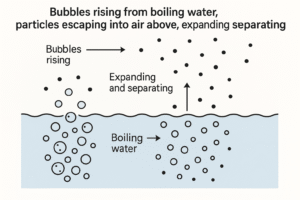 Liquid → Gas
Liquid → Gas
When you keep heating a liquid, the particles gain even more energy — enough to completely overcome the forces holding them together.
-
They break free and spread out to become a gas.
-
Boiling happens at a specific temperature called the boiling point.
Example: Water boiling into steam at 100°C
Evaporation
 Liquid → Gas (slowly)
Liquid → Gas (slowly)
Evaporation is a slower process that happens at any temperature, not just the boiling point. It starts at the surface of the liquid.
-
A few high-energy particles escape into the air.
-
Over time, the liquid gradually disappears.
Example: A puddle drying up on a warm day
Condensation
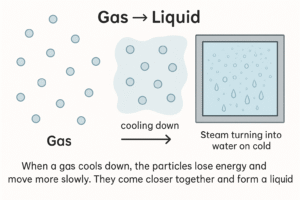 Gas → Liquid
Gas → Liquid
When a gas cools down, the particles lose energy and move more slowly. They come closer together and form a liquid.
-
This is the reverse of boiling or evaporation.
Example: Steam turning into water on a cold window
Sublimation
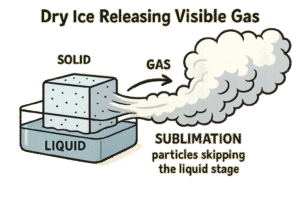 Solid → Gas (without becoming liquid)
Solid → Gas (without becoming liquid)
Some substances can go straight from a solid to a gas — skipping the liquid stage. This is called sublimation.
-
It only happens with specific materials like dry ice.
Example: Dry ice (solid carbon dioxide) turning into gas
Deposition
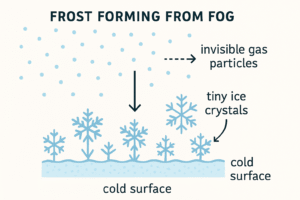 Gas → Solid (without becoming liquid)
Gas → Solid (without becoming liquid)
The reverse of sublimation — a gas becomes a solid directly, usually in cold conditions.
Example: Frost forming on windows from water vapour
Now that we’ve seen all the state changes, let’s put it all together in a simple diagram you can remember for revision.
Interconversion of States – With Diagram
We’ve talked about melting, boiling, freezing, and more. But how do all these state changes fit together?
Chemists often use a simple interconversion diagram to show how matter moves between solid, liquid, and gas — and what causes those changes.
The key idea is this:
Heating adds energy to particles. Cooling removes energy.
Let’s look at how that works in all directions
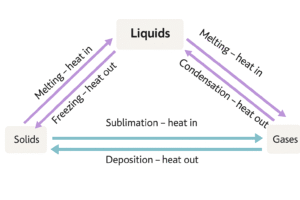
Solid to Liquid → Melting
-
Heat is added
-
Particles gain energy and break from fixed positions
Liquid to Solid → Freezing
-
Heat is removed
-
Particles lose energy and form a regular structure
Liquid to Gas → Boiling or Evaporation
-
More heat is added
-
Particles gain enough energy to fully escape
Gas to Liquid → Condensation
-
Heat is removed
-
Particles slow down and come closer
Solid to Gas → Sublimation
-
Heat is added
-
Particles jump directly from solid to gas (e.g. dry ice)
Gas to Solid → Deposition
-
Heat is removed quickly
-
Particles go straight from gas to solid (e.g. frost)
Why This Diagram Matters
This kind of diagram is often tested in GCSE exams. You should be able to:
-
Label each change correctly
-
Know which processes add energy (heating)
-
Know which ones remove energy (cooling)
-
Recognise examples of each
You don’t need to memorise every temperature — just understand the direction of energy change and what it does to particle movement.
Next, let’s apply this idea to the real world — by looking at how different materials behave at room temperature.
Temperature and State at Room Conditions
We’ve seen that matter can change state when it’s heated or cooled. But what about normal conditions — like in a classroom or at home?
At room temperature (around 25°C), substances can be in any of the three states depending on their melting and boiling points.
Here’s how it works:
-
If the room temperature is below a substance’s melting point, it stays a solid
-
If it’s between the melting and boiling point, it’s a liquid
-
If it’s above the boiling point, it becomes a gas
Let’s look at some examples:
| Substance | Melting Point | Boiling Point | State at Room Temp |
|---|---|---|---|
| Water | 0°C | 100°C | Liquid |
| Oxygen | –219°C | –183°C | Gas |
| Iron | 1538°C | 2862°C | Solid |
| Ethanol (Alcohol) | –114°C | 78°C | Liquid |
| Carbon Dioxide | –78°C (sublimes) | — | Gas |
As you can see, the same rules apply to every material — what matters is how its melting and boiling points compare to the surrounding temperature.
Mini Diagram: Temperature vs State
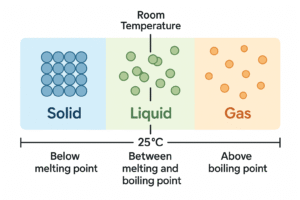
Understanding this concept helps explain simple things like:
-
Why water is a liquid at room temperature
-
Why oxygen is a gas
-
Why metals like iron stay solid unless heated to extreme temperatures
This also helps you answer exam questions where you’re asked to predict the state of a substance at a given temperature.
Key Takeaways
Let’s recap the most important things you’ve learned about the states of matter:
-
Matter is anything that has mass and takes up space
-
All matter is made of tiny particles with space between them
-
The arrangement and movement of particles determine whether a substance is a solid, liquid, or gas
-
Solids have tightly packed, vibrating particles
-
Liquids have close, flowing particles
-
Gases have widely spaced, fast-moving particles
-
Heating gives particles more energy → leads to melting, boiling, or sublimation
-
Cooling removes energy → leads to freezing, condensation, or deposition
-
Changes of state are physical changes — the substance stays the same
-
A substance’s state at room temperature depends on its melting and boiling points
Understanding these basics helps you build a strong foundation for future topics like bonding, chemical reactions, and real-world applications in food, air, and materials.
Glossary
Matter – Anything that has mass and takes up space
Particle Theory – The idea that all matter is made up of tiny, constantly moving particles with space between them
Kinetic Energy – The energy particles have due to their movement
Intermolecular Forces – The invisible forces of attraction between particles
Melting Point – The temperature at which a solid becomes a liquid
Boiling Point – The temperature at which a liquid becomes a gas
Sublimation – When a solid turns directly into a gas without becoming liquid first
Deposition – When a gas turns directly into a solid
Condensation – When a gas cools and becomes a liquid
Evaporation – A slow process where a liquid becomes gas from the surface
Diffusion – The spreading of particles from an area of high concentration to low concentration
F&Q
What are the 3 states of matter?
The three main states of matter are solids, liquids, and gases. Each state has unique particle arrangements and behaviors.
What is the particle theory of matter?
The particle theory explains that all matter is made up of tiny particles that are constantly moving, with spaces between them.
Is change of state a chemical change?
No, it’s a physical change. The substance remains the same but changes form.
What state is water at room temperature?
Water is a liquid at room temperature (25°C) because it is between its melting point (0°C) and boiling point (100°C).
read more
How did scientist first discover water is made of Oxygen and Hydrogen?

Leave a Reply