Introduction In our previous blog, we explained the basics of electrolysis and how electricity can drive chemical changes that do not happen on their own. In this post, we will look at one of the simplest and most important examples: the electrolysis of water. This school experiment is a clear demonstration of how water, which …
What Is Electrolysis of Water?
Introduction
In our previous blog, we explained the basics of electrolysis and how electricity can drive chemical changes that do not happen on their own. In this post, we will look at one of the simplest and most important examples: the electrolysis of water.
This school experiment is a clear demonstration of how water, which looks like a single, simple liquid, is actually made of two elements — hydrogen and oxygen. By passing an electric current through water, we can split it into these gases and collect them in their natural 2:1 ratio.
This experiment not only helps students understand electrolysis better but also shows the link between chemical equations and what we can observe in real life.
What Is Electrolysis of Water?
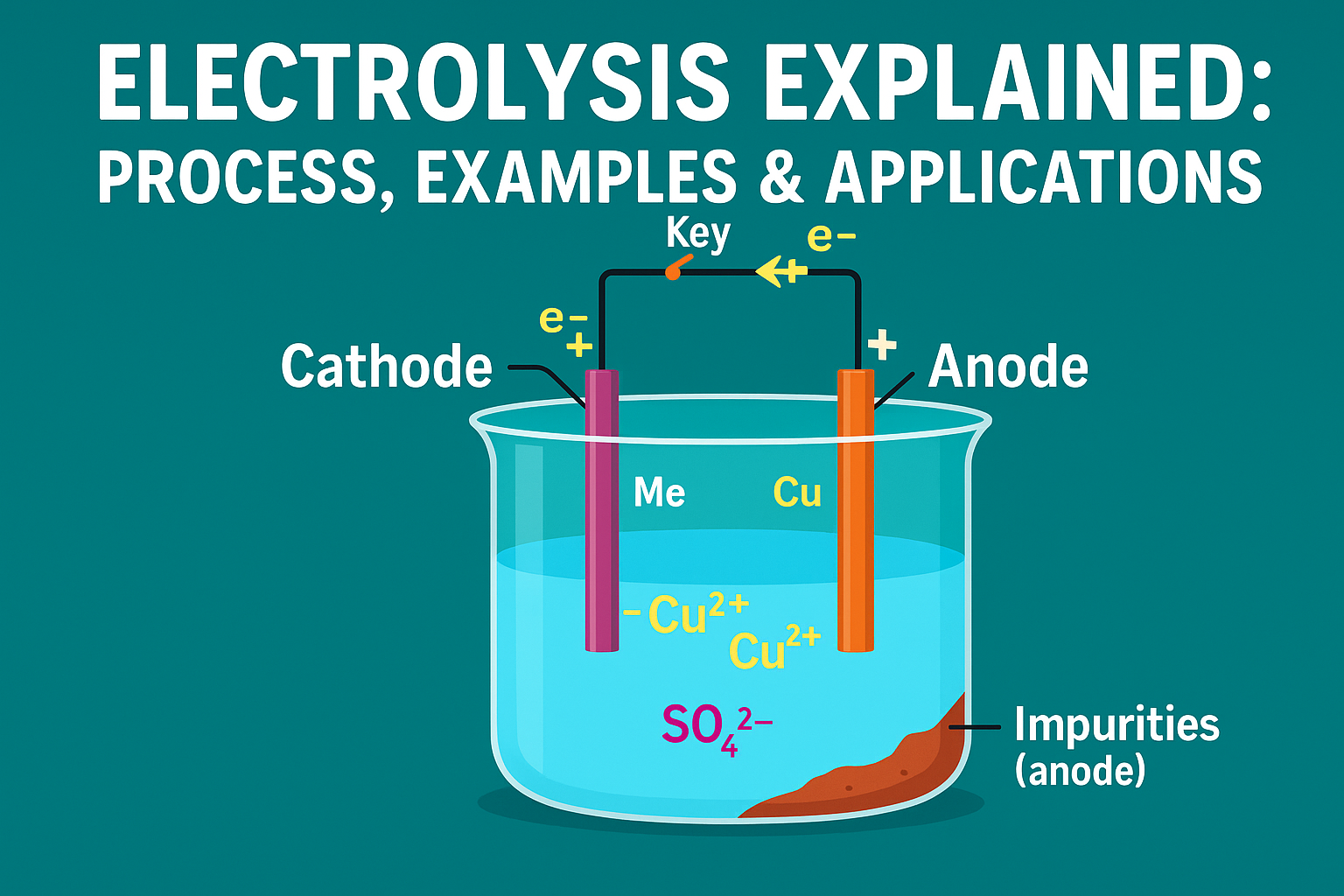
Water is a compound made of hydrogen and oxygen, with the chemical formula H₂O. Each water molecule contains two hydrogen atoms joined to one oxygen atom. On its own, pure water does not conduct electricity well, so a small amount of an electrolyte (such as dilute sulfuric acid) is usually added to help the process.
When an electric current is passed through the water, the molecules are split into their elements. Hydrogen gas is released at the cathode (negative electrode), and oxygen gas is released at the anode (positive electrode). The volume of hydrogen collected is always about twice the volume of oxygen, which matches the formula H₂O.
This makes the electrolysis of water a powerful way to show that water is not just a single substance but a combination of two different elements.
[Image: Simple diagram showing electrolysis of water setup | Alt text: “Diagram of electrolysis of water with hydrogen and oxygen gases collected”]
Special Note: Why Is Water Dangerous with Electricity if It Is a Poor Conductor?
Pure water on its own does not conduct electricity well. However, the water we come across in daily life (tap water, rainwater, river water) is never pure. It contains dissolved salts, minerals, and impurities, which make it a good conductor of electricity.
That is why it is extremely dangerous to touch water when it is in contact with live electrical wires or devices. The dissolved ions allow electricity to pass through the water — and through your body if you come into contact with it.
So while electrolysis experiments are safe in controlled conditions with low-voltage batteries, real-life situations involving water and mains electricity can be deadly.
Materials Needed for the Experiment
To carry out the electrolysis of water in a school lab, you only need a few simple items:
-
Beaker – filled with distilled water.
-
Electrolyte – a few drops of dilute sulfuric acid (or sometimes sodium hydroxide) to help the water conduct electricity.
-
Electrodes – two graphite or other inert electrodes.
-
Battery or power supply – usually a low-voltage DC source (like a 6–12 V battery).
-
Wires and clips – to connect the electrodes to the battery.
-
Test tubes – placed upside down over each electrode to collect the gases.
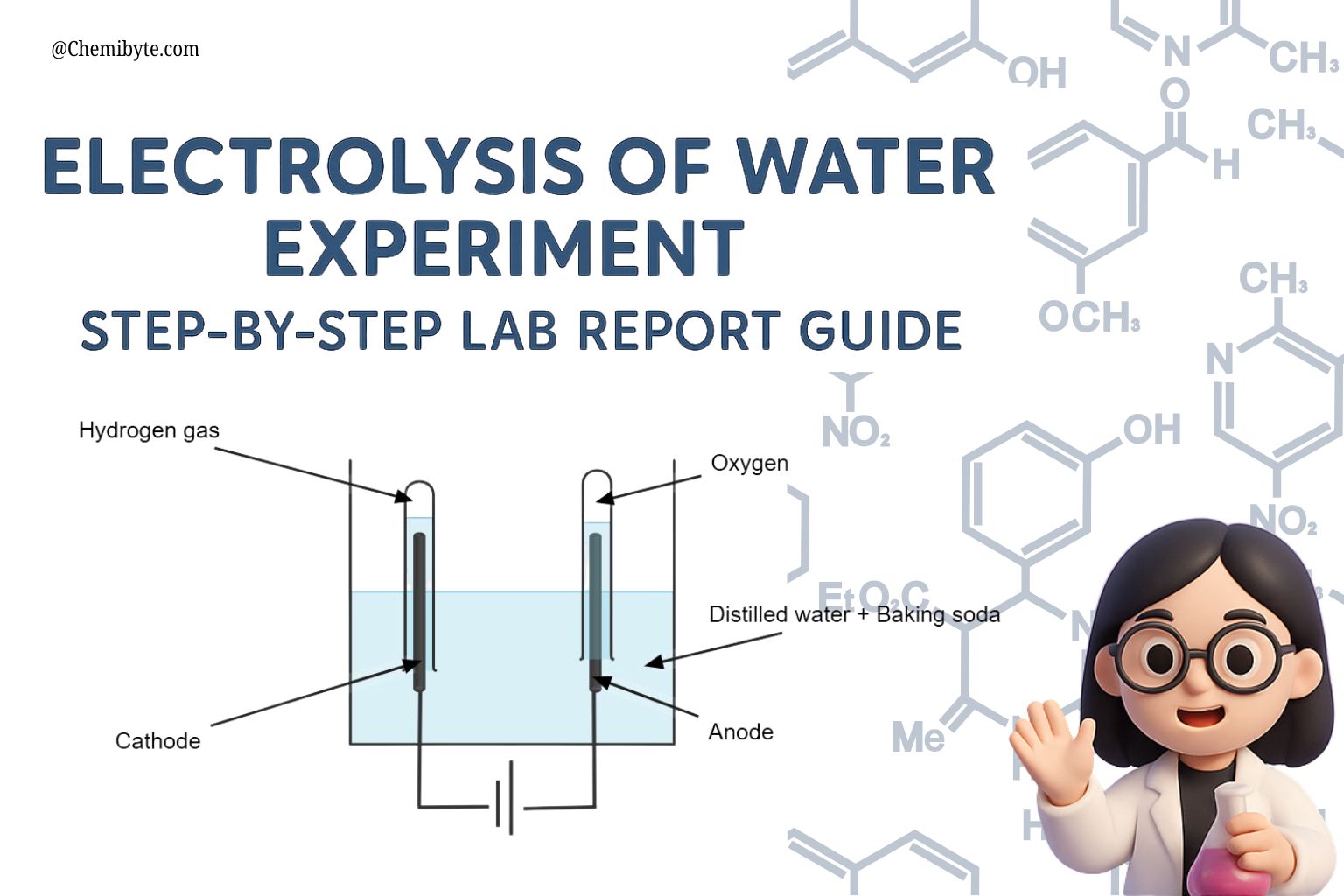
Step-by-Step Method
-
Set up the apparatus: Fill a beaker with distilled water and add a few drops of dilute sulfuric acid to act as the electrolyte.
-
Insert the electrodes: Place the two graphite (or inert) electrodes into the water, making sure they do not touch each other.
-
Prepare to collect gases: Fill two test tubes with water and carefully invert them over the electrodes so they stay full of water.
-
Connect the circuit: Attach the electrodes to a low-voltage DC power supply (battery).
-
Switch on the current: Turn on the power supply and watch closely.
-
Observe the bubbles: Gas bubbles will begin to form at both electrodes, gradually displacing the water inside the test tubes.
What Do You Observe?
-
Bubbles of gas form at both electrodes.
-
The test tube connected to the cathode (negative electrode) fills up faster.
-
The test tube connected to the anode (positive electrode) fills up more slowly.
-
When you compare them, you will notice that twice as much gas is collected at the cathode compared to the anode.
This is because:
-
The gas at the cathode is hydrogen (H₂).
-
The gas at the anode is oxygen (O₂).
So the ratio of gases collected is about 2:1, which matches the chemical formula of water, H₂O.
Explanation of the Chemistry
Water (H₂O) is made of hydrogen and oxygen. During electrolysis, electricity forces these atoms to separate and form gases. Here is what happens step by step:
At the Cathode (Negative Electrode)
-
Hydrogen ions (H⁺) from the water gain electrons (reduction).
-
They combine to form hydrogen gas (H₂).
2H++2e−→H22H⁺ + 2e⁻ → H₂2H++2e−→H2
At the Anode (Positive Electrode)
-
Hydroxide ions (OH⁻) from the water lose electrons (oxidation).
-
They form oxygen gas (O₂) and water.
4OH−→O2+2H2O+4e−4OH⁻ → O₂ + 2H₂O + 4e⁻4OH−→O2+2H2O+4e−
Overall Reaction
2H2O(l)→2H2(g)+O2(g)2H₂O(l) → 2H₂(g) + O₂(g)2H2O(l)→2H2(g)+O2(g)
This means that for every 2 molecules of water, we get 2 molecules of hydrogen gas and 1 molecule of oxygen gas. That’s why you always collect twice as much hydrogen as oxygen.
Real-World Applications of Water Electrolysis
While the school experiment is small-scale, the same principle is used in industry and technology:
1. Hydrogen Fuel Production
Electrolysis is used to make hydrogen gas, which can be stored and later used in hydrogen fuel cells to power cars, buses, and even rockets.
2. Oxygen Supply
The oxygen released during electrolysis can be used in hospitals, welding, and steel-making industries where high-purity oxygen is needed.
3. Chemical Industry
Hydrogen from electrolysis is used to make ammonia for fertilizers and to turn vegetable oils into margarine (hydrogenation).
4. Clean Energy
Electrolysis of water is one way to store renewable energy from solar or wind power, by converting it into hydrogen fuel that can be used later.
[Image: Industrial setup of water electrolysis | Alt text: “Industrial electrolysis of water producing hydrogen and oxygen gases”]
Precautions to Take During the Experiment (and Why They Are Important)
Even though the electrolysis of water is a safe and simple school experiment, it involves electricity, acids, and gases. Here are the main precautions and the reasons behind them:
-
Use a low-voltage power supply (6–12 V only)
-
High voltage can cause electric shocks or overheating of the wires. A small battery is safe but still strong enough to split water.
-
-
Add only a few drops of dilute sulfuric acid
-
Pure water does not conduct electricity well, so we add a little acid to help. But if too much acid is used, the solution becomes corrosive and unsafe to handle.
-
-
Handle the test tubes carefully when collecting gases
-
The gases are light and escape quickly. Rough handling may cause breakage, spillage, or loss of results.
-
-
Keep flames away from the experiment
-
Hydrogen is highly flammable. If a flame or spark is nearby, it can ignite and cause a small explosion.
-
-
Wear safety goggles
-
Even dilute acid can irritate the eyes. Bubbles or splashes from the solution can be harmful if they come in contact with your face.
-
-
Wash your hands after the experiment
-
This ensures that no traces of acid or chemicals remain on your skin, which could cause irritation later.
-
By following these precautions, students can carry out the experiment safely while still observing the fascinating chemistry at work.
Conclusion / Key Takeaways
The electrolysis of water is one of the simplest but most powerful experiments in school chemistry. With just a battery, a beaker of water, and two electrodes, we can see water split into its elements — hydrogen and oxygen — in the exact 2:1 ratio predicted by its formula, H₂O.
This experiment helps students understand that:
-
Electricity can cause chemical changes (electrolysis).
-
Water is a compound made of hydrogen and oxygen.
-
The gases produced have real uses in fuel, industry, and medicine.
-
Safety precautions are always essential in practical chemistry.
By linking classroom theory with visible results, this practical makes the abstract idea of chemical equations much easier to grasp.
How to Confirm the Gases Produced
During the electrolysis of water, you collect two gases — hydrogen and oxygen. To confirm which is which, simple test-tube experiments are used:
Test for Hydrogen (Cathode Gas)
-
Collect the gas from the cathode in a test tube.
-
Bring a lighted splint close to the mouth of the tube.
-
If the gas is hydrogen, it burns with a ‘pop’ sound.
-
This is known as the ‘pop test’ for hydrogen.
Test for Oxygen (Anode Gas)
-
Collect the gas from the anode in a test tube.
-
Insert a glowing splint (a splint with its flame just blown out but still glowing).
-
If the gas is oxygen, the splint relights.
-
This confirms the presence of oxygen.
These two simple tests are widely used in schools to identify gases safely.
Conclusion / Key Takeaways
The electrolysis of water is one of the most important school experiments because it shows, in a very simple way, how electricity can split a compound into its elements. By using a small battery and two electrodes, we can see water decompose into hydrogen and oxygen in a 2:1 ratio, exactly as predicted by its formula H₂O.
Through simple gas tests, we can also confirm the identity of the products:
-
Hydrogen makes a ‘pop’ sound with a lighted splint.
-
Oxygen relights a glowing splint.
This experiment helps students connect what they learn in theory — chemical equations and atomic ratios — with what they can see in real life. It also highlights how these gases are valuable in industry, fuel production, and medicine.
Most importantly, it reminds us of the role of safety in chemistry: using low voltage, handling acids carefully, and keeping flames away from hydrogen. With these precautions, the electrolysis of water becomes a safe, exciting, and unforgettable classroom demonstration.
Questions & Answers on Water Electrolysis Experiment
Q1. Why do we add a few drops of dilute sulfuric acid to the water?
A: Pure water is a poor conductor of electricity. The dilute sulfuric acid provides ions, which allow the current to pass through and make electrolysis possible.
Q2. Why is more gas collected at the cathode than at the anode?
A: Twice as much hydrogen is produced compared to oxygen because the formula of water is H₂O. Each molecule contains 2 atoms of hydrogen for every 1 atom of oxygen.
Q3. How do we test for the gases produced?
A: Hydrogen makes a ‘pop’ sound when tested with a lighted splint, while oxygen relights a glowing splint.
Q4. Why is hydrogen produced at the cathode?
A: The cathode is negatively charged, so positively charged hydrogen ions (H⁺) move towards it, gain electrons, and form hydrogen gas.
Q5. Why must we keep flames away from the experiment?
A: Hydrogen is very flammable. If a flame is nearby, it can catch fire or even cause a small explosion.
Q6. What is the overall equation for the electrolysis of water?
A:
2H2O(l)→2H2(g)+O2(g)2H₂O(l) → 2H₂(g) + O₂(g)2H2O(l)→2H2(g)+O2(g)
Extra Application Questions
Q7. Suppose you collected 16 g of oxygen gas during the experiment. How many grams of hydrogen would you expect to collect?
A: The ratio of hydrogen to oxygen in water is 2:1 by atoms.
-
Molar mass of oxygen (O₂) = 32 g/mol
-
Molar mass of hydrogen (H₂) = 2 g/mol
From the balanced equation:
2H2O→2H2+O22H₂O → 2H₂ + O₂2H2O→2H2+O2
1 mole of oxygen (32 g) is produced with 2 moles of hydrogen (4 g).
So if 16 g of oxygen is collected, the hydrogen should be:
(4÷32)×16=2g(4 ÷ 32) × 16 = 2 g(4÷32)×16=2g
Q8. How does this result prove that water is made of hydrogen and oxygen in a 2:1 ratio?
A: The experiment shows that for every 1 part by volume of oxygen collected, there are 2 parts by volume of hydrogen. This matches the molecular formula H₂O — two hydrogen atoms for every one oxygen atom.
Challenging Questions
Q9. Draw and label a neat diagram of the electrolysis of water experiment.
A: Students should draw:
-
A beaker with water + dilute sulfuric acid.
-
Two electrodes connected to a battery (DC supply).
-
Inverted test tubes over each electrode to collect gases.
-
Labels: Cathode (negative, hydrogen collected), Anode (positive, oxygen collected).
(Teachers often give marks for correct labels + neat diagram.)
Q10. During an experiment, a student collected 40 cm³ of oxygen gas. Predict the volume of hydrogen gas collected and explain your reasoning.
A: From the formula of water (H₂O), hydrogen and oxygen are produced in a 2:1 ratio.
-
If oxygen = 40 cm³, hydrogen should be 80 cm³.
-
Explanation: For every 1 molecule of oxygen released, 2 molecules of hydrogen are released.
Q11. Why is it important to use inert electrodes (like graphite) instead of metal electrodes in this experiment?
A: Metal electrodes may react with the products or take part in side reactions, which would interfere with the results. Inert electrodes (graphite, platinum) do not react, so they allow only water to decompose into hydrogen and oxygen.
Subscribe to Our Newsletter
Keep in touch with our news & offers